C2h4 Lewis Acid Or Base
Soft Lewis acids and bases are relatively large polarizable atoms ions and molecules. Predicted data is generated using the US Environmental Protection Agencys EPISuite.

Which Of The Following Is Not A Lewis Acid A Sif4 B C2h4 C Bf3 D F
It is a chemical formula for Ethylene or Ethene.

C2h4 lewis acid or base. Log Kow KOWWIN v167 estimate 127 Log Kow Exper. Lewis acids and bases are described by the Lewis theory of acid-base reactions as electron-pair acceptors and electron pair donors respectively. The purposes of this work are 1 to characterize the nature of Lewis acidbase interaction in Cp 2 MLHBF 3 AlF 3 adducts M V Nb Ta.
Lewis acids and bases are described by the Lewis theory of acid-base reactions as electron-pair acceptors and electron pair donors respectively. C2h4 is a lewis base because because it has sufficient elecctron pair and can therefore donte electrons in a covalent bond. But as implied earlier it can also be a Lewis base.
CH4 methane is lewis base What is an acid base neutral. Therefore ion act as Lewis acid as it accepts electron pairs from the oxalate ion which is a Lewis base. When aluminum chloride is under discussion it is called a Lewis acid or an electrophile.
3 to evaluate the effect of various types of ligands on the MLHBAl bonds. 1995 Boiling Pt Melting Pt Vapor Pressure Estimations MPBPWIN. Aluminum chloride AlCl3 is a Lewis acid because the aluminum atom has an open valence shell.
Is sicl4 a Lewis acid. Sana maintindihan niyo. Database match 113 Exper.
Is alcl3 a Lewis acid. An illustration detailing the reaction between a Lewis acid and base leading to. A Lewis base then is any species that has a filled orbital containing an electron pair which is not involved in bonding but may form a dative bond with a Lewis acid to form a Lewis adduct.
A base is a substance that donates an unshared pair of electrons to a recipient species with which the electrons can be shared. The whole HCl molecule is. In practice soft acids prefer to associate with soft bases and hard acids prefer to associate with hard bases.
Is Oh Lewis acid or base. A Lewis acid is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. Hence according to Lewis concept these are Lewis acids.
C2h4 h2o Lewis acid and Lewis base - 15691591 belsanjose01 belsanjose01 04062021 Science Senior High School answered C2h4 h2o Lewis acid and Lewis base 1 See answer kaiisenpaii kaiisenpaii Explanation. Is C2H4 a Lewis base. B C2H4 ExplanationIn BF3 and FeCl3 molecules the central atoms have incomplete octet and in SiF4 the central atom has empty d-orbitals.
Now sicl4 is able to keep greater than 8 electrons in its outermost orbit. L CO C 2 H 4 PCH 3 3. Log Octanol-Water Partition Coef SRC.
So it takes electron pairs from other compounds which have. You can eliminate the. Since carbon monoxide has a method for accepting electrons it by definition can be a Lewis acid.
C2h4 is a lewis base because because it has sufficient elecctron pair and can therefore donte electrons in a covalent bond Aswanth 18 Points. Therefore a Lewis base can donate a pair of electrons to a Lewis acid to form a product containing a coordinate covalent bond. A Lewis base is any substance such as the OH- ion that can donate a pair of nonbonding electrons.
2 to determine the influence of M V Nb and Ta on MLHBAl interactions. Is hydrochloric acid a Lewis acid. The correct option is.
For example NH3 is a Lewis base because it can donate its lone pair of electrons. C2H4 Lewis Structure Ethylene Welcome to Geometry of Molecules and today in this video we are going to help you know the step-by-step method for determining the Lewis Structure of C2H4 molecule. According to Lewis An acid is a substance that accepts a pair of electrons and in doing so forms a covalent bond with the entity that supplies the electrons.
Hard Lewis acids and bases are relatively small and less polarizable. Trimethylborane is a Lewis acid. This product is also referred to as a Lewis adduct.

Is C2h4 Polar Or Nonpolar All About C2h4 Polarity

Structures Of The C2h2 Hf I C2h4 Hf Ii And C3h6 Hf Iii Download Scientific Diagram

Molecular Graphs Of Electron Density L Co A C2h4 B P Ch3 3 Download Scientific Diagram

Is C2h4 Polar Or Nonpolar All About C2h4 Polarity
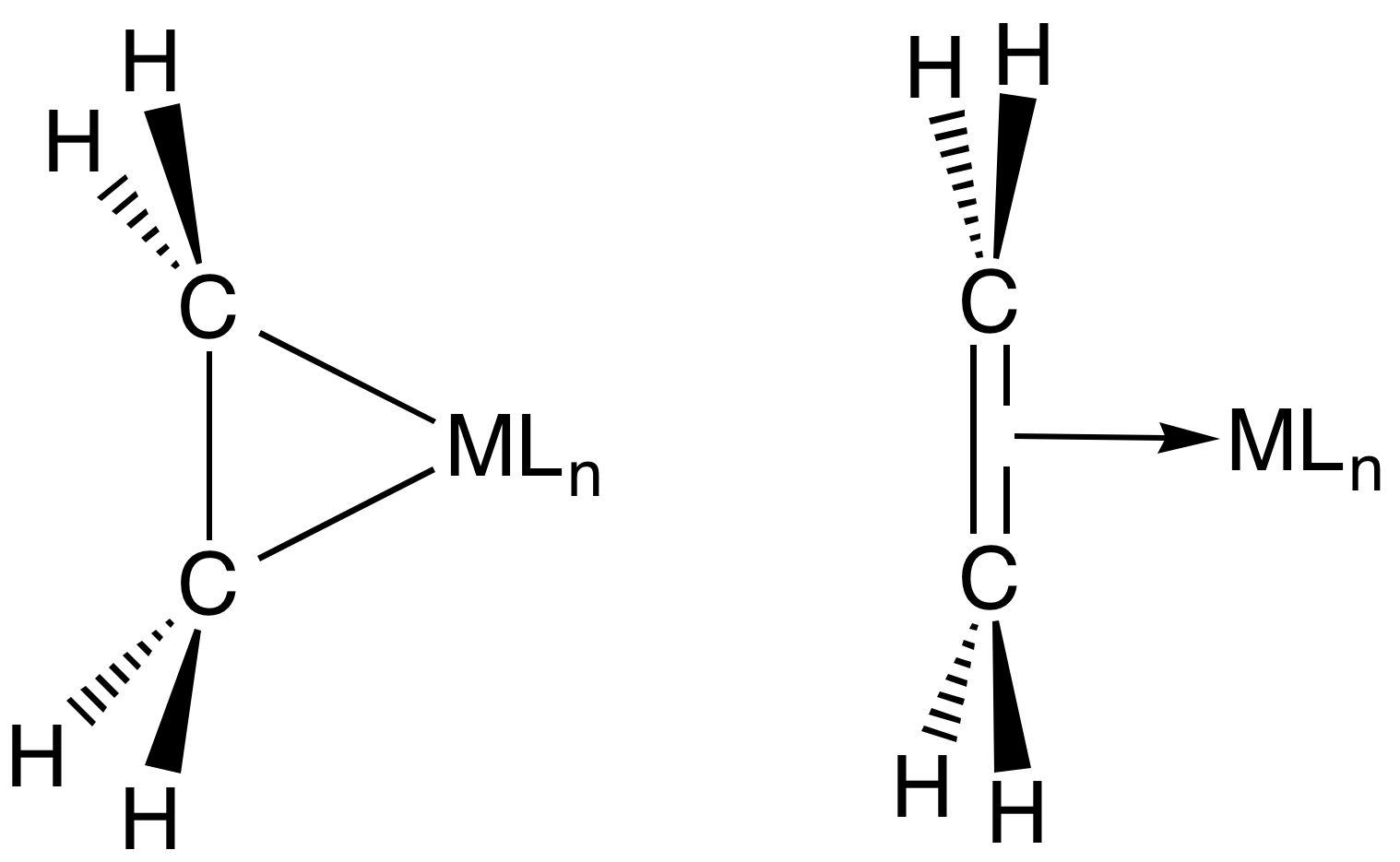
Transition Metal Alkene Complex Wikiwand

Oxidative Dehydrogenation Of Ethane Common Principles And Mechanistic Aspects Gartner 2013 Chemcatchem Wiley Online Library

Is C2h4 Polar Or Nonpolar All About C2h4 Polarity

Structures Of The C2h2 Hf I C2h4 Hf Ii And C3h6 Hf Iii Download Scientific Diagram

Which Of The Following Is Not A Lewis Acid A Sif4 B C2h4 C Bf3 D F

The Molecular Graphs Of The Alf3 C2h2 The Top Left Albr3 C2h4 The Download Scientific Diagram

Is C2h4 Polar Or Nonpolar All About C2h4 Polarity
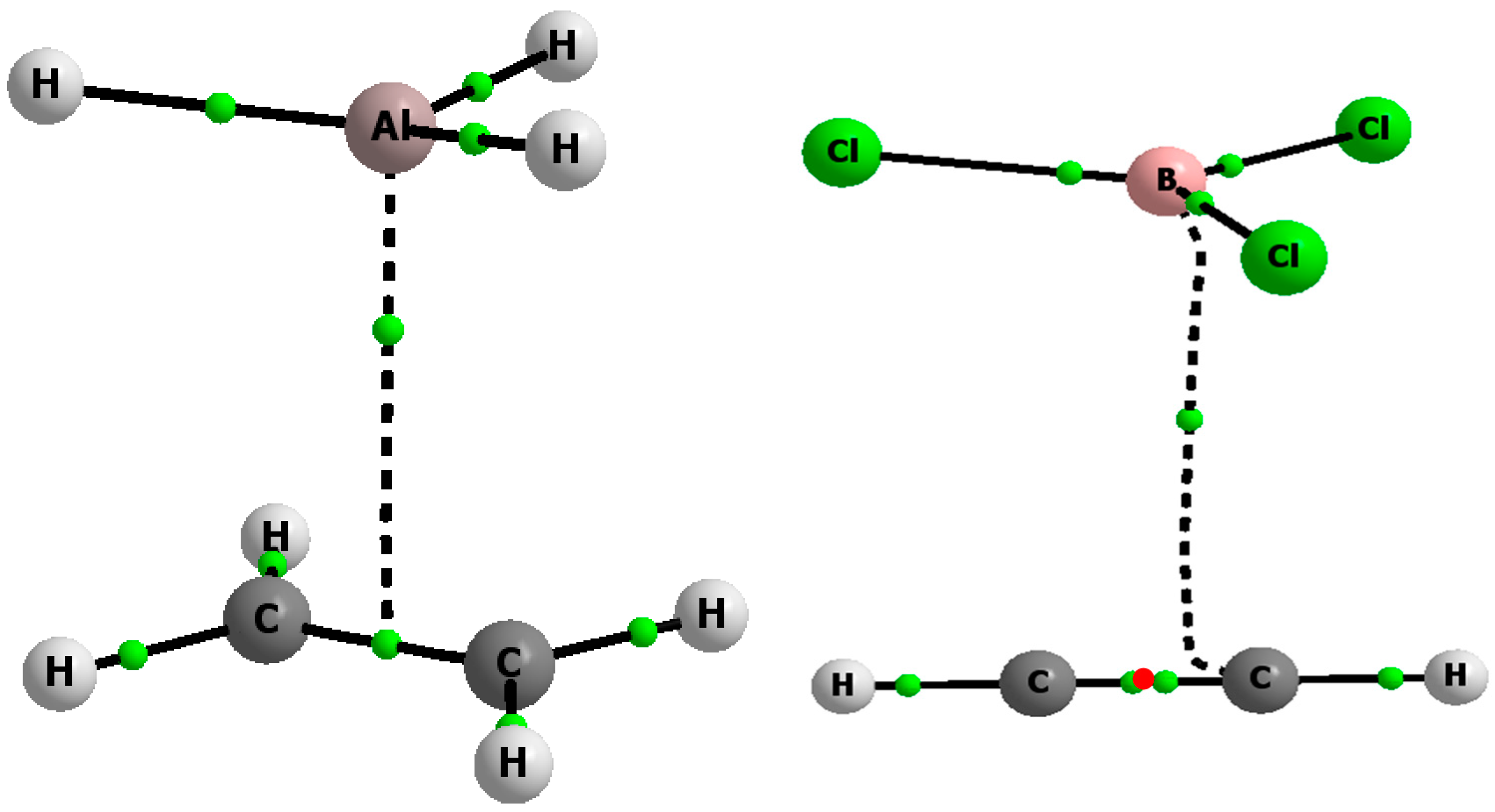
Molecules Free Full Text Triel Bonds P Hole P Electrons Interactions In Complexes Of Boron And Aluminium Trihalides And Trihydrides With Acetylene And Ethylene Html

What Are Hydrocarbons Gulf Coast Environmental Systems Chemistry Organic Chemistry Chemistry Classroom
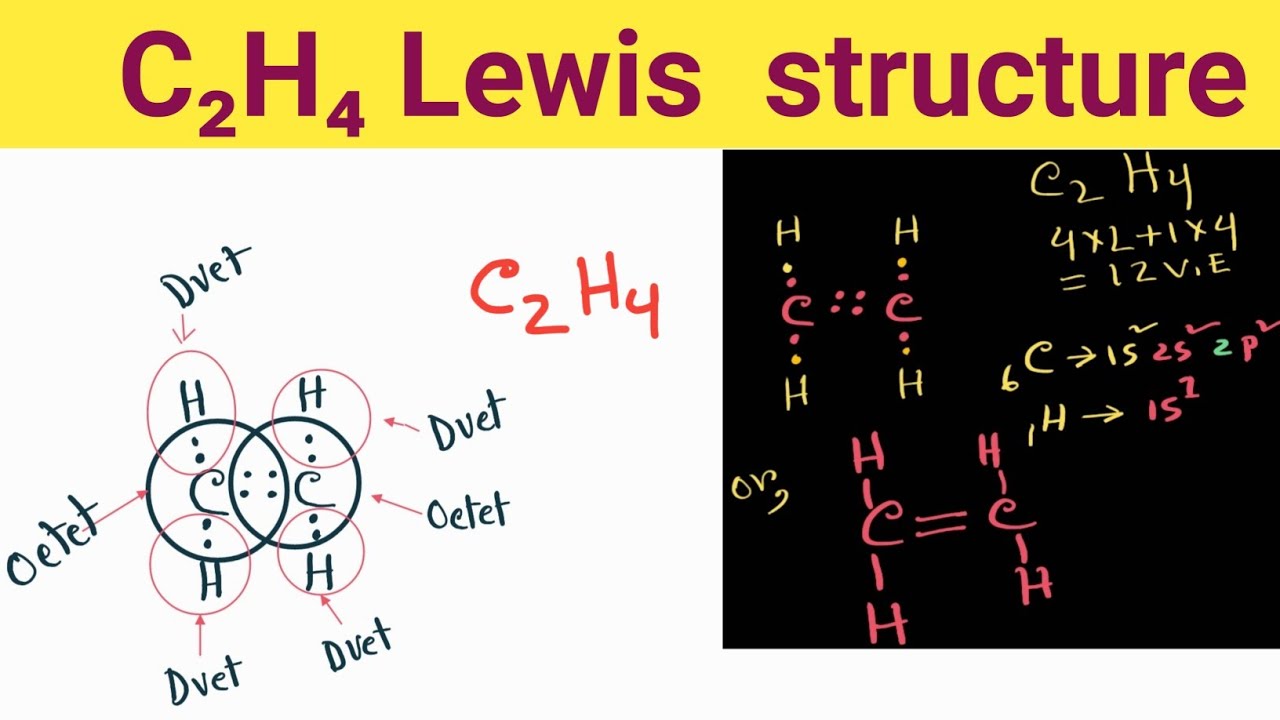
C2h4 Lewis Structure How Do You Draw The Lewis Structure For C2h4 Youtube
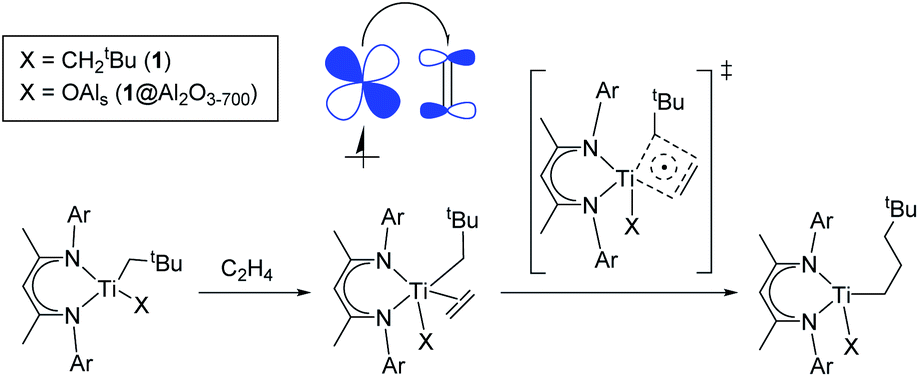
Molecular And Supported Ti Iii Alkyls Efficient Ethylene Polymerization Driven By The P Character Of Metal Carbon Bonds And Back Donation From A S Chemical Science Rsc Publishing Doi 10 1039 D0sc04436a

Molecular Graphs Of Electron Density L Co A C2h4 B P Ch3 3 Download Scientific Diagram

Experimental Microwave Mw Structure Of Complex C2h4 Ag Cl Download Scientific Diagram
