Draw The Lewis Structure Of C2h4. How Many Lone Pairs Are There
Draw the Lewis structures of the given molecules. Lewis dot structure of C 2 H 4 Alternatively a dot method can be used to draw the lewis structure.

C2h4 Lewis Structure Molecular Or Electron Geometry Polar Or Nonpolar
How many lone pairs are there.

Draw the lewis structure of c2h4. how many lone pairs are there. Drawing the Lewis Structure for ClO-. Subtract step 3 number from step 1. Draw the Lewis structures of the given molecules.
Drawing the Lewis structure for C2H4 named ethene requires the use of a double bond. How many single bonds are there. How many double bonds are there.
For the ClO- Lewis structure the total number of valence electrons found on the periodic table for the ClO- molecule. The lone pair is also called the unshared pair. What are the steps to draw Lewis structures.
Use the Rest of the Dots to Finish Your Lewis Structure. Therefore only nine 12-3 9 valence electrons pairs are remaining. Find number of bonds by diving the number in step 3 by 2 because each bond is made of 2 e- 10e-2 5 bond pairs.
Drawing the Lewis structure for C2H4 named ethene requires the use of a double bond. First mark those nine valence electrons pairs as lone pairs in outside atoms on oxygen atoms. What is the charge of ClO 4-Charge of ClO 4-ion is -1.
Use the atomic symbol for your answer. According to the lewis structure of Ethene there is no lone pair present on the central atom. Remaining oxygen atom has three lone pairs and ceenter atom chlorine does not has lone pairs.
Draw Two Electrons Between Atoms to Form the Chemical Bonds. Lone pairs on atoms. 4 bonds x 2 8 8 lone pairs x 2 16 Total is 24.
22-10 12e-6 lone pairs. There are three ways we could draw this or three resonance hybrids for carbonate. This -1 negative charge is located at an oxygen atom.
There are two triangles overlapping each other as we can see in the diagram. According to the VSEPR chart the shape of the ethene molecule is trigonal planar. Which of the following ions does not have a charge of exactly -2.
O 2 bonds 2 lone pairs or O 1 bond 3 lone pairs. In a double bond two pairs of valence electrons are shared for a total of four valence electrons. How many lone pairs are there.
How many single bonds are there. How many dots should be drawn in the Lewis Dot Structure of AsH3. LP VE.
Find the Number of lone pairs present on the central atom of the C2H4 Lewis structure. Next Three Questions Apply To The Lewis Structure Of Ethane C2H4 How Many Double Bonds Are There In The Lewis Structure Of C H. Which atom is more electronegative oxygen or sulfur.
In a double bond two pairs of valence electrons are shared for a total of four valence electrons. The Lewis dot structure of C2H4 C 2 H 4 shows that there are four electrons shared between the two carbon atoms and two electrons are shared between each carbon and hydrogen. Draw the Lewis structure of NH3.
How many lone pairs are there. Trigonal Planar O T-shaped Seesaw Tetrahedral Octahedral How Many Bonding Electrons Are There. OF CO2 Select Draw Rings More Erase Select Draw Rings More Erase.
12-12 0e-0 lone pairs Use information from step 4 and 5 to draw the lewis structure. 3 O 1 2 4 What Is The Molecular Geometry Of C2H4. Draw the Lewis structure of C2F4.
Use information from step 4 and 5 to draw the lewis structure. Draw the Lewis structure of C2H4. Drawing the Lewis Structure for C2H For C2H4 you have a total of 12 total valence electrons.
Check that each atom except hydrogen has four electron pairs. Draw the Lewis structure of CF4. Placing the Elements in the Drawing.
Replace two lone pairs with one bond. There are three oxygen atoms which are connected through double bonds to chlorine atom. Each of those oxygen atoms have two lone pairs.
In last shells there are nine lone pairs on atoms. Find the number of nonbonding lone pairs e-. 4 3 2 O 1 How Many Lone Pairs Of Electrons Are There On The Central Atom Of C2H4.
Once we know how many valence electrons there are in ClO- we can distribute them around the central atom with the goal of filling the outer shells of each atom. To confirm it you can also use the formula to find lone pairs. Include lone pairs on all atoms where appropriate.
Find the Total Number of Valence Electrons refer to the Instructions Below the Pictures Step 2. Chemistry questions and answers. There are already three C-O bonds in the above sketch.
Draw the Lewis structure of C2H4. For C2H4 In C2H4 if we look into the lewis structure we will see that there are three bonded pairs of electrons around each carbon and zero lone pair. Drawing the Lewis Structure for C2H For C2H4 you have a total of 12 total valence electrons.

Lewis Dot Structure Easy Hard Science

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

Lewis Dot Structure Easy Hard Science
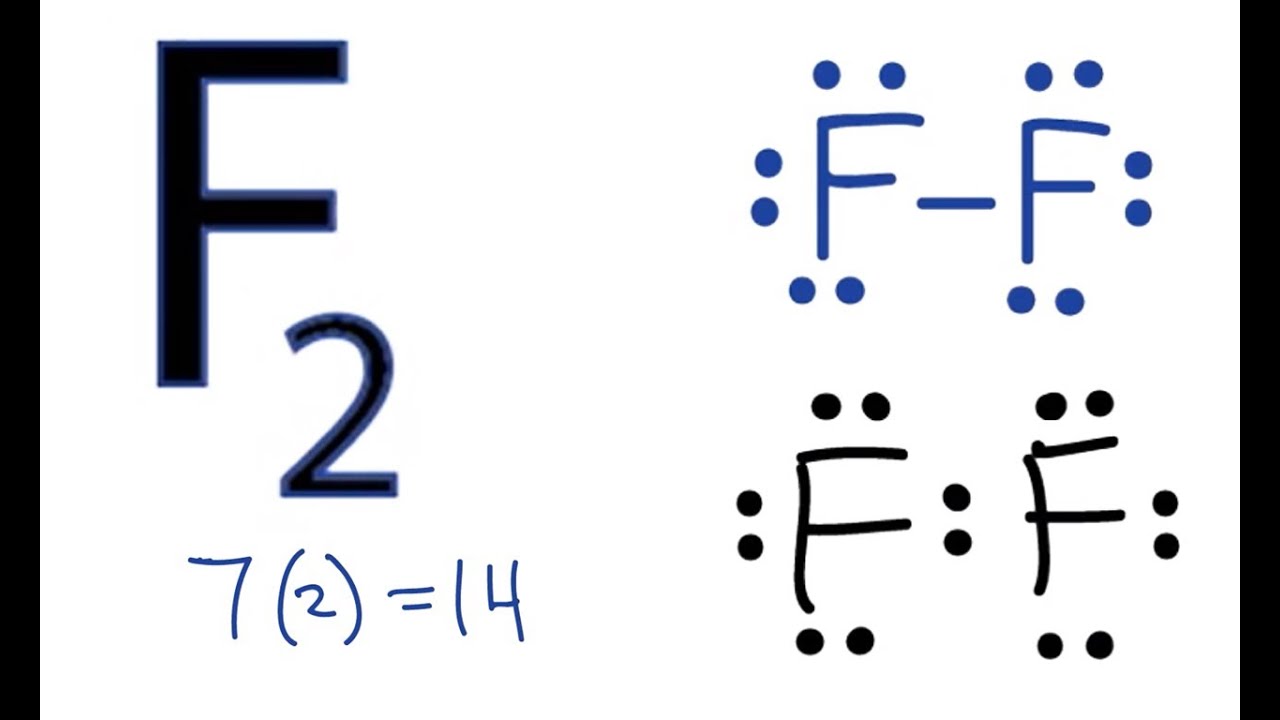
F2 Lewis Structure How To Draw The Lewis Dot Structure For F2 Youtube
Ethene C2h4 Lewis Structure Hybridization

Ccl4 Lewis Structure How To Draw The Dot Structure For Ccl4 Carbon Tetachloride Youtube

Draw The Electron Dot Structure Of Ethene C2h4 Brainly In
Ethene C2h4 Lewis Structure Hybridization

Lewis Dot Structure Easy Hard Science

C3h4 Lewis Structure How To Draw The Lewis Structure For Ch3cch Youtube

Methane Molecule Showing Covalent Bonding Dot And Cross Covalent Bonding Structural Formula Molecules

Lewis Dot Structure Easy Hard Science

Lewis Dot Structure Easy Hard Science

Lewis Dot Structure Easy Hard Science

C2h4 Molecular Geometry Shape And Bond Angles Youtube

From Gen Chem To Org Chem Pt 7 Lewis Structures Master Organic Chemistry Organic Chemistry Molecular Geometry Organic Chemistry Books

Average Values Of C C Bonds Depending On Hybridization Bond Length Chemistry Bond

