So3 2- Lewis Structure With Formal Charges
Youll want to calculate the formal charges on each atom to make sure you have the best Lewis structure for SO 3. EBook 0-s0 Print References Multiple Choice.
.jpg)
Lewis Structure For So32 Sulfite Ion Resonance Structures
Sulfur atom is the center atom and four oxygen atoms are located around sulfur atom.
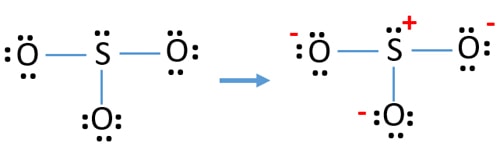
So3 2- lewis structure with formal charges. A Lewis structure for SO3 that obeys the octet rule showing all non-zero formal charges is shown he many resonance structures for SO3 that obey the octet rule are possible. It is determined by the number of electrons an atom brought number of lone pair of electrons half the number of electrons in bond formation. The molecule SO3 is triangular with resonance.
1 2 3 4 In structure 1 the formal charges are 2 for S and 1 for both O atoms. The Lewis structure consists of an SO double bond and two SO dative bonds without utilizing d-orbitals. First the valence electrons are placed around the carbon atom.
This is an old chestnutwe gots 24 valence electrons to distribute. Draw the Lewis dot structure that have resonance and do not expand the octet. Lewis structure of SO 4 2-There are two SO bonds and two S-O bonds in sulfate ion lewis structure.
The electrical dipole moment of gaseous sulfur trioxide is zero. SeO 3 selenium trioxide. I also go over hybridization shape and.
Because of equal formal charge distribution throughout the atom double covalent bonds form in SO3. Show all equivalent resonance structures and on any one of the resonance structures show the formal charge on each atom. The Lewis structure consists of an SO double bond and two SO dative bonds without utilizing d-orbitals.
Youll need to place a double bond on the Oxygen atom in order to have the best Lewis structure. The formal charges of the SO 2 with the single bond and a double bond is larger than the SO 2 with two double bonds. In the Lewis structure of CH3I the formal charge on the terminal hydrogen atom is zero.
The low formal charges of S make structures 2 and 3 more stable or more important contributors. Draw the Lewis dot structure that minimizes the formal charges. Lewis Dot of the Sulfite Ion.
You might think youve got the correct Lewis structure for SO 3 at first. There is more equal charge distribution which further stabilizes the structure. When you draw the ion with all single bonds and 3 lone pairs on each oxygen there is a formal charge of 1 on S and -1 on each O as shown.
So the formal charge of Oxygen is 0. In order to calculate the formal charges for SO3 2- well use the equationFormal charge of valence electrons - nonbonding val electrons - bonding e. Incorrect sulfite ionpng However when you delocalize the oxygen lone pairs and form a double bond the charge is decreased so the molecule becomes more stable.
Now first we will find formal charge of sulfur S S 6 0 122 0 S 0. So the formal charge of sulfur is 0. The ion SO3 2- is pyramidal.
The Lewis Structure for sulfite has 3 resonance structures in which the Sulfur has a formal charge of 0 and two of the oxygens have a formal charge of -1. SO3 SeO2 Nakladatel Its a good idea to check the formal charges for your SeO 2 Lewis structure to make sure they are zero. So for oxygen it is 6-6-1.
There are no lone pairs in the last shell of sulfur atom. The Lewis structure for SO 3 2-is requires you to place more than 8 valence electrons on Sulfur S. The sulfite anion SO3 2- is present in wines and is used as preservative in certain foods.
Wiki User 2011-01-24 002811. The Lewis Dot Structure for SO 3 2-. We could write SO_3 or Ostackrel2S-O_2- All of these structures are equivalent and the similarly the corresponding acid of SO_3 H_2SO_4 has an ambiguous Lewis structure.
In terms of electron-counting formalism the sulfur atom has an oxidation state of 6 and a formal charge of 2. Formal charge valence electrone non bonding valence electrone bonding electrone2. What is the formal charge of SO3 2.
For the SO3 2- Lewis structure the total number of valence electrons. Remember Sulfur is in Period 3 and can hold more than 8 valence electrons. Now we will find O O 6 4 42 0 O 0.
In this post we discussed the method to construct the CH3I Lewis structure. In structures 2 and 3 the formal charges are 1 for S and 1 for the oxygen atom with a single bond to S. Draw the Lewis dot structure that minimizes the formal charges and expands the octet.
The bonds between the selenium and the oxygen are double bonds. Formal charge on hydrogen atom of CH3I molecule 1- 0-22 0. Now we will find the formal charge of SO3 by using this formula.

Lewis Structure For So32 Sulfite Ion Resonance Structures

How To Calculate The Formal Charges For So3 2 Sulfite Ion Youtube

Co32 Lewis Structure How To Draw The Lewis Structure For Co3 2 Carbonate Ion Youtube
How Is The Hybridization Of So3 2 Determined Quora

How Many Electron Dots Are In The Lewis Structure Of So 3 2 Study Com
What Are All Resonance Structures For So3 Socratic
How To Calculate The Formal Charges For So3 2 Sulfite Ion Youtube
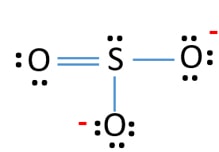
Lewis Structure For So32 Sulfite Ion Resonance Structures

Draw The Lewis Structure For The So32 Clutch Prep

So3 Lewis Structure Molecular Geometry And Hybridization Techiescientist
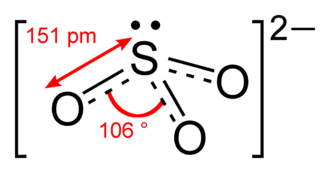
What Is The Difference In Structure Of So3 And So3 2 Socratic

So3 2 Lewis Structure How To Draw The Lewis Structure For So3 2 Sulfite Ion Youtube

What Is The Difference Between The Lewis Structure Of So3 Vs So3 2 Quora

So3 2 Lewis Structure How To Draw The Lewis Structure For So3 2 Sulfite Ion Youtube
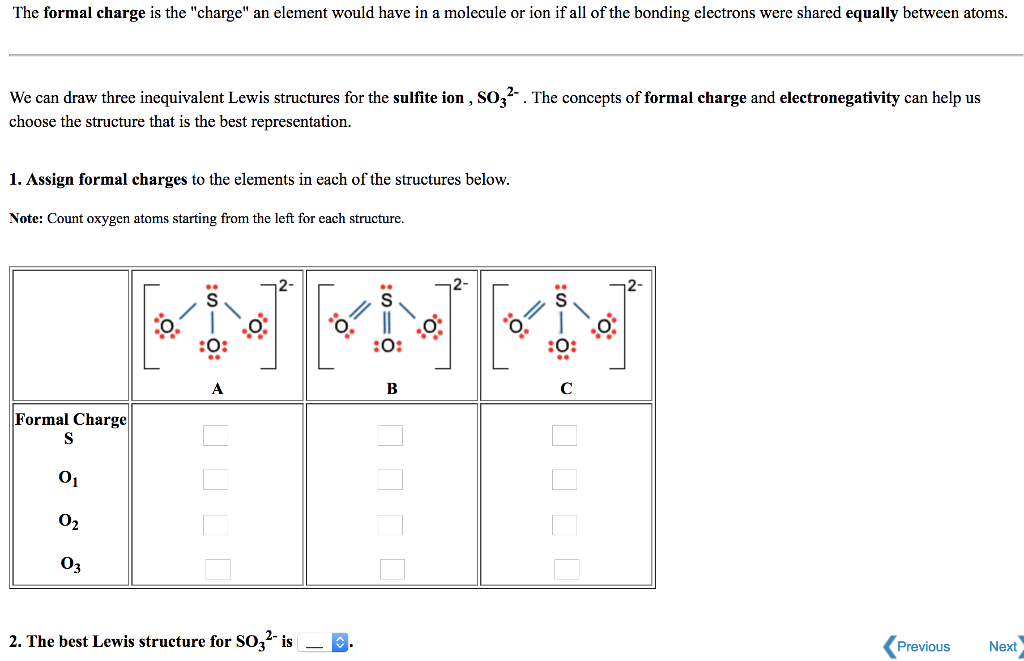
The Formal Charge Is The Charge An Element Would Chegg Com

So3 2 Lewis Structure Sulfite Ion Youtube
Lewis Structure For So32 Sulfite Ion Resonance Structures

So3 2 Lewis Structure How To Draw The Lewis Structure For So3 2 Sulfite Ion Youtube

How Many Different Types Of Resonance Stru Clutch Prep