The Lewis Structure Of Hcn Contains
More Related Question Answers. 1 Show answers Another question on Chemistry.

Makethebrainhappy The Lewis Dot Structure For Hcn
After determining how many valence electrons there are.

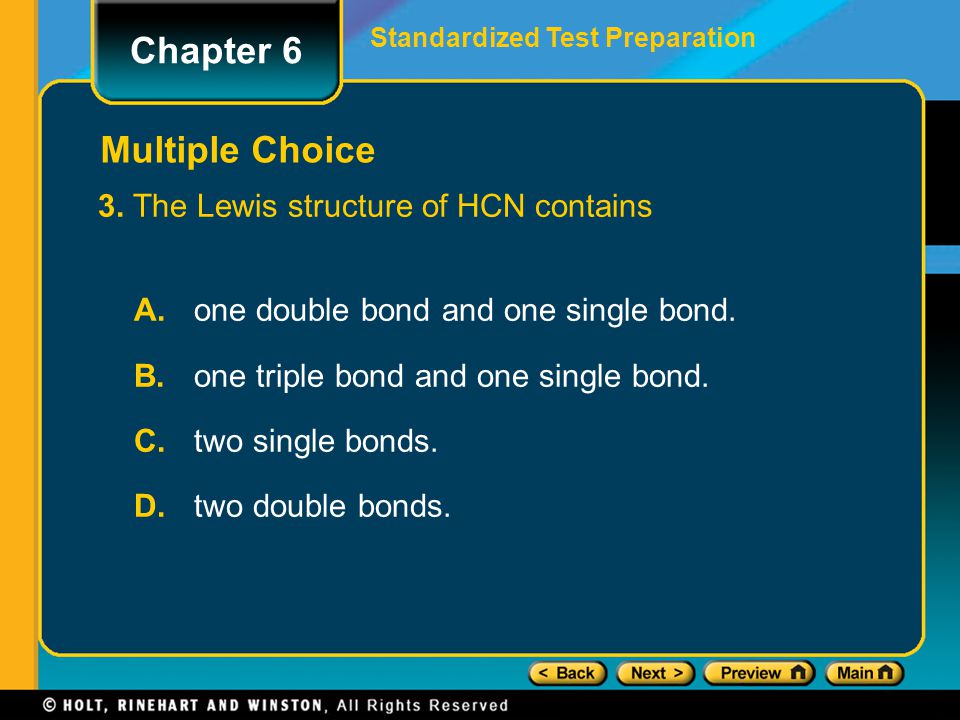
The lewis structure of hcn contains. The Lewis structure of HCN contains _______. 6e- 2 x 7e- 20e- H C N HCN. The Lewis structure of hypochlorous acid has oxygen O with single bonds between hydrogen and chlorine.
The Lewis structure of HCN contains A. Continue to order Get a quote. What are the charges of the subatomic particles by choosing the answer from the drop down menu.
In the Lewis structure we see that hypochlorous acid has 14 valence electrons. Continue to order Get a quote. The lewis structure of hcn contains a one double bond and one single bond b one triple bond and one single bond c two single bonds d two double bonds.
O 2 F OF 2 6e - 2 x 7e - 20e - H C N HCN. Once you get the total number of valence electrons you can make a Lewis dot structure of HCN. Sulfuric acid a component of acid rain reacts with limestone calcium carbonate to produce calcium sulfate and carbon dioxide.
One triple bond and. One of the following contains a double bond as well as single bonds. Two single bondsDtwo double bonds.
What does bond length exactly mean. The lewis structure of hcn contains a one double bond and one single bond b one triple bond and one single bond c two single bonds d two double bonds. The Lewis structure of HCN contains A.
Four are used as bonding electrons and the remaining 10 are nonbonding electrons on oxygen and chlorine. One double bond and one single bond. It also aids with understanding the bonds formed in the molecule and the electrons not participating in any.
Put least electronegative atom in centre3. One double bond and one single bond. HCN is used in electroplating mining and as a precursor for several compounds.
This is the total number of electrons that must be used in the Lewis structure. 1 Get Other questions on the subject. The Lewis structure of HCN containsAone double bond and one single bondBone triple bond and one single bondC.
O 2 F OF 2. If 300 g of titanium metal is reacted with 600 g of chlorine gas cl2 to form 77 g of titanium iv chloride in a combination reaction what is the. A covalent chemical compound contains a certain arrangement of valence electrons involving the connected atoms as described by a Lewis structure.
How many pi bonds and sigma bonds are there in the tetracyanoethylene molecule. Is HOCl a Lewis acid. Hydrogen carbon and nitrogen.
One double bond and one single bond. The lewis structure of hcn contains a one double bond and one single bond b one triple bond and one single bond c two single bonds d two double bonds. A one double bond and one single bond b one triple bond and one single bond c two single bonds d two double bonds.
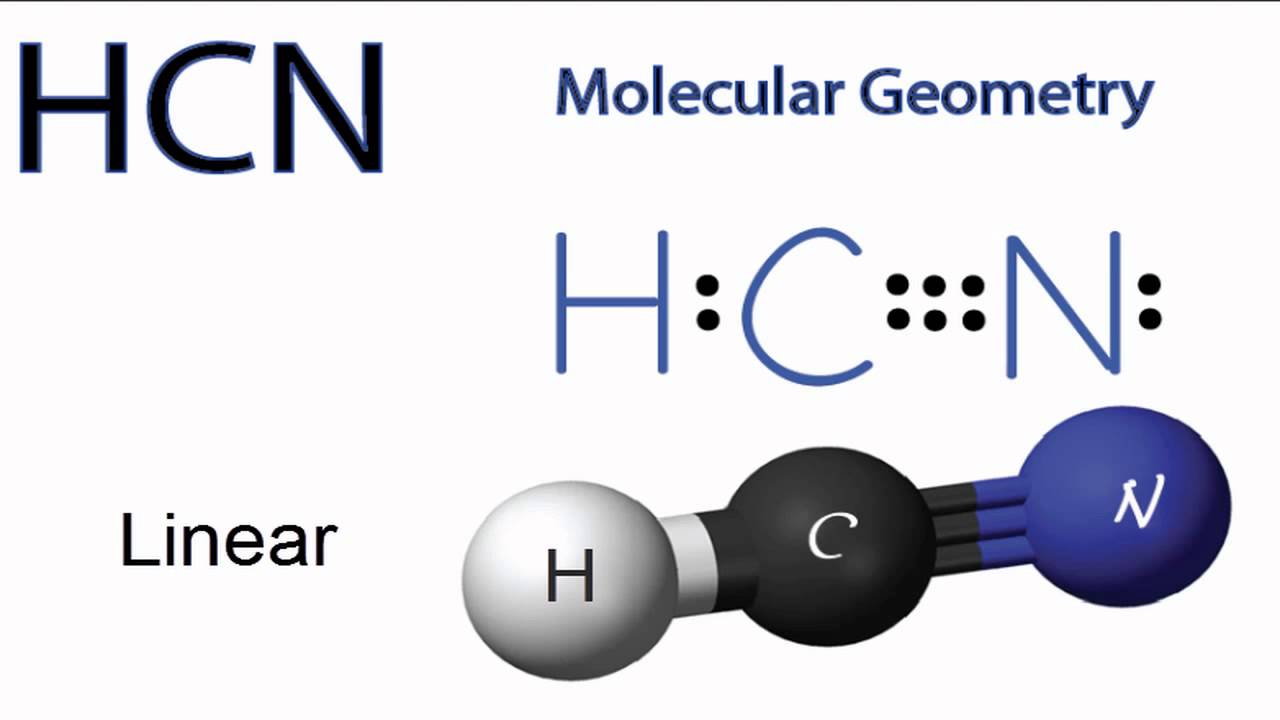
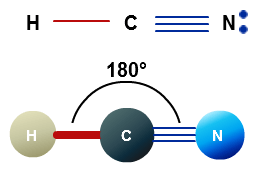
Hence Hydrogen Cyanide HCN has ten valence electrons. It is a polar molecule with bond angles of 180 degrees. One triple bond and.
Note that H and F can only form one bond and are always on the periphery. 1e- 4e- 5e- 10e-2. Chemistry 22062019 0130 kaliloabousjbf.
HCN Lewis structure comprises three different atoms. The Lewis Structure Lewis Dot Diagram for HCN1. Which element would mostly likely have an electron affinity measuring closest to zero.
Protons have a 10or-1. The lewis structure of hcn contains a one double bond and one single bond b one triple bond and one single bond c two single bonds d two double bonds. The Lewis structure of HCN contains _____.
One triple bond and. The Lewis structure of HCN contains A. This is the total number of electrons that must be used in the Lewis structure.
Drawing the Lewis Structure for HOCl This means that even though. The Lewis structure of HCN containsAone double bond and one single bondBone triple bond and one single bondC. For the HCN Lewis structure calculate the total number of valence electrons for the HCN molecule.
1 Show answers Another question on Chemistry. 1 Show answers Another question on Chemistry. This browser does not support the video element.
This structure helps in understanding the arrangement of valence electrons around the atoms in the molecule. A one double bond and one single bond b one triple bond and one single bond c two single bonds d two double bonds. Two single bondsDtwo double bonds.
Draw a skeleton structure of the molecule or ion arranging the atoms around a central atom and connecting each atom to the central atom with a single one electron pair bond. Put one electron pair in each. Gadolinium oxide a colorless powder which absorbs carbon dioxide from the air.

Draw The Lewis Dot Structure Of Hydrogen Cyanide Hcn Molecule
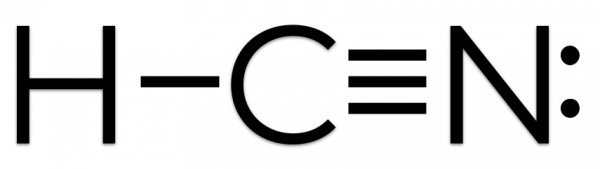
Hcn Lewis Structure Molecular Geometry Shape And Polarity
Chapter 6 Preview Multiple Choice Short Answer Extended Response Ppt Video Online Download

Chapter 8basic Concepts Of Chemical Bonding Prof Dr

Chemistry Semester 2 Final Exam Review Questions Ppt Download

Hcn Lewis Structure Molecular Geometry Shape And Polarity

Is Hcn Polar Or Nonpolar Techiescientist

Hcn Lewis Structure How To Draw The Lewis Structure For Hcn Youtube

Makethebrainhappy The Lewis Dot Structure For Hcn

Lewis Structures Simplified For Studying Physical Science Ap Chemistry Chemistry

Hcn Lewis Structure How To Draw The Lewis Structure For Hcn Youtube
Hcn Lewis Structure Molecular Geometry Shape And Polarity

Chapter 6 Objectives Section 1 Introduction To Chemical Bonding Ppt Download

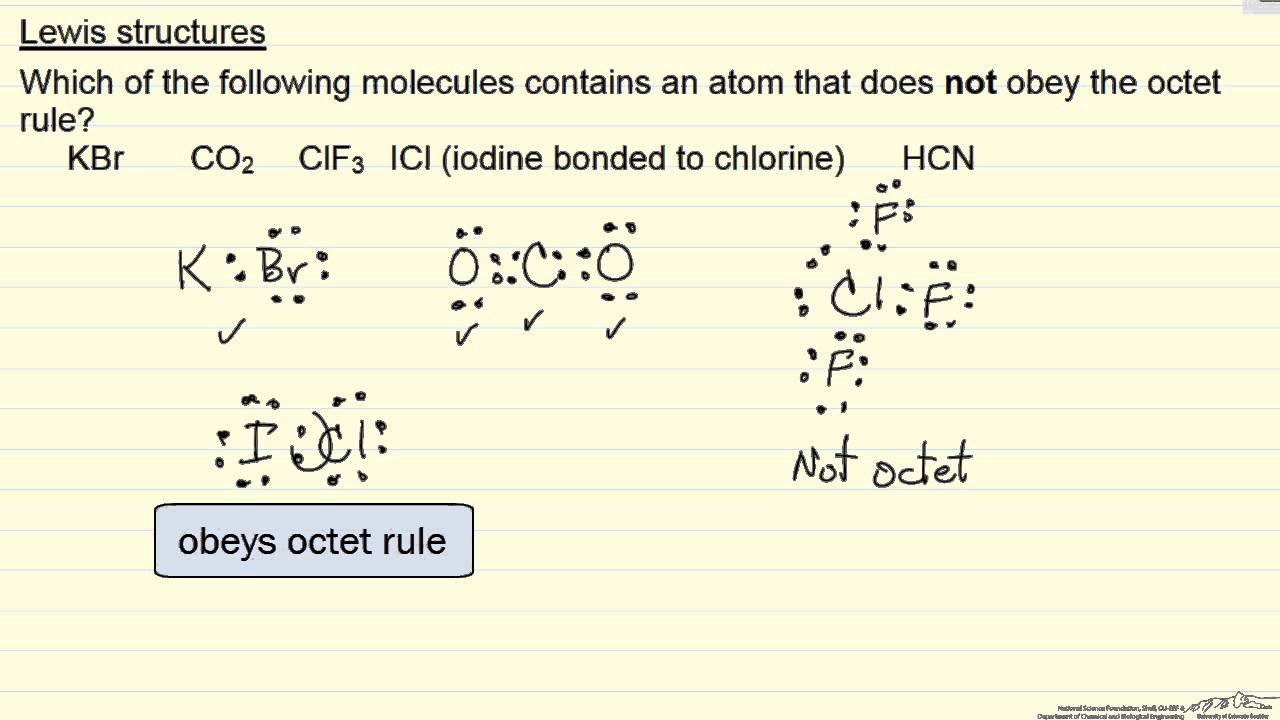
Lewis Structures Octet Rule Example Youtube
Hcn Molecular Geometry Youtube
Hcn Lewis Structure Molecular Geometry Shape And Polarity
Lewis Electron Dot Structures Ck 12 Foundation

Hcn Lewis Structure Molecular Geometry Shape And Polarity
Lewis Structures Octet Rule Example Youtube
