Scl2 Lewis Structure Electron Geometry
None of these require pi-bonding which is the method of formation for double and triple bonds. Each Chlorine atom has six valence electrons after the bonds are formed.
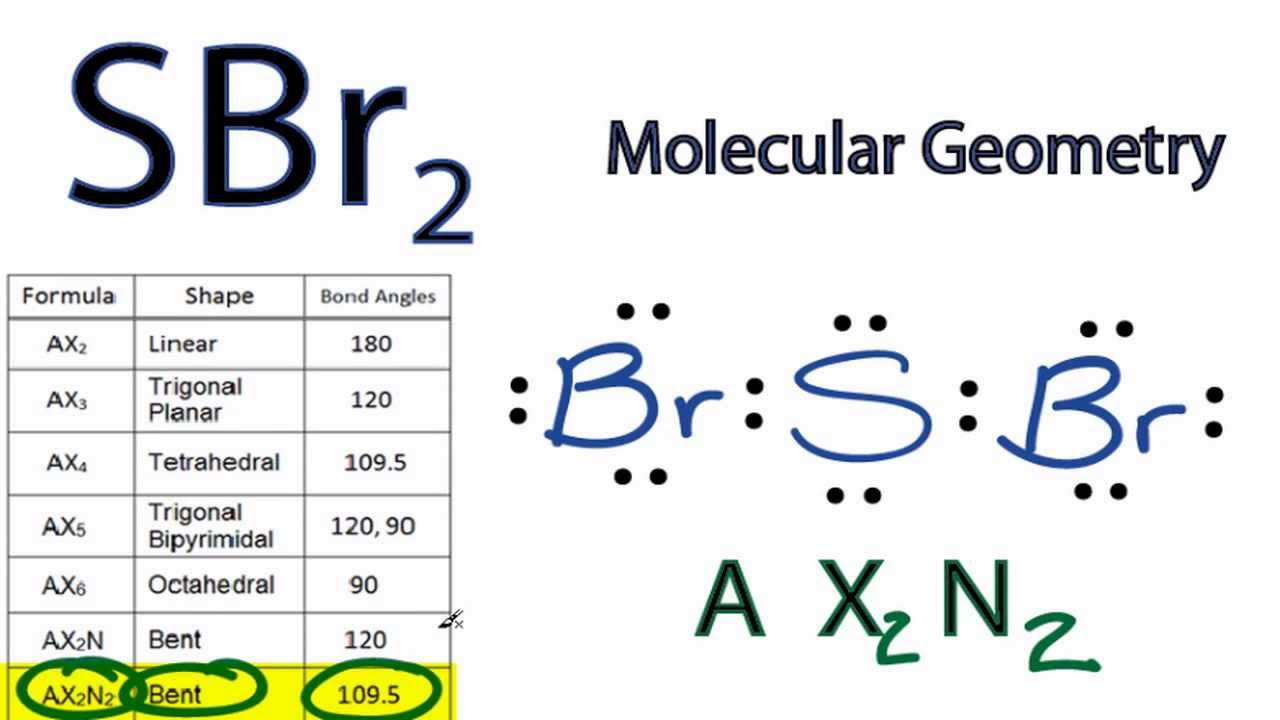
Sbr2 Molecular Geometry Shape And Bond Angles Youtube
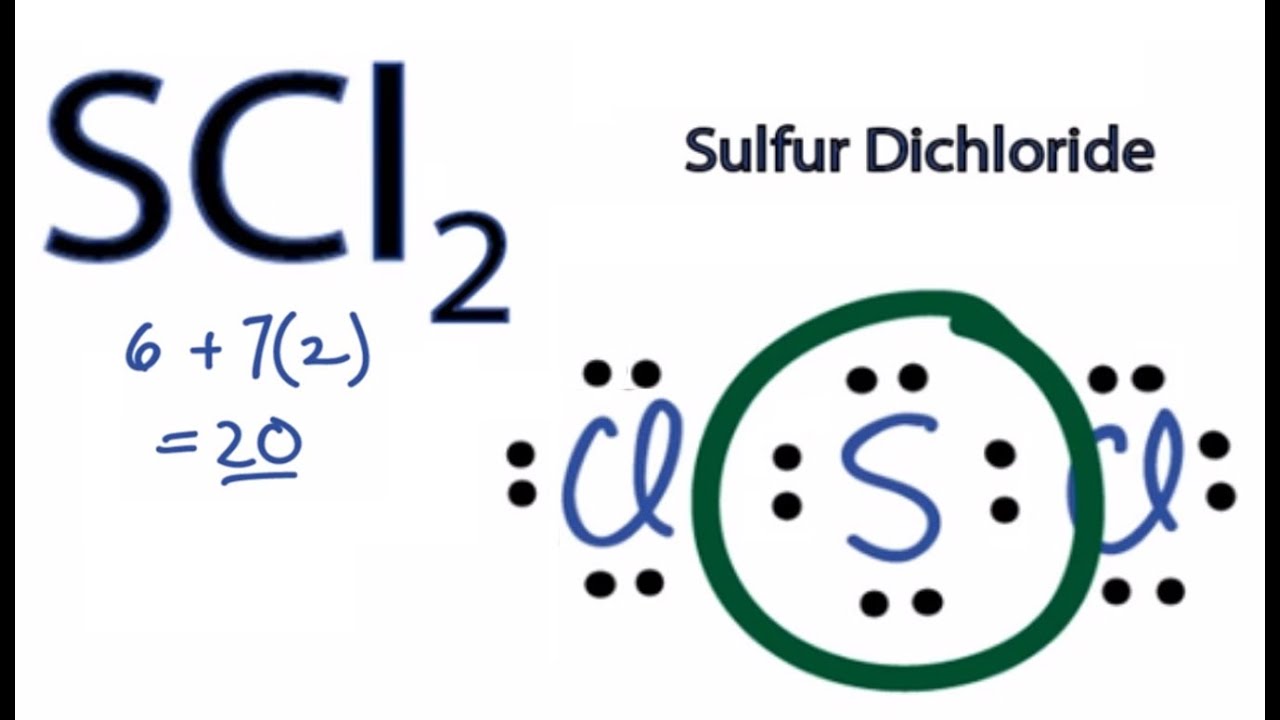
For the SCl2 Lewis structure we have a total of 20 valence electrons.
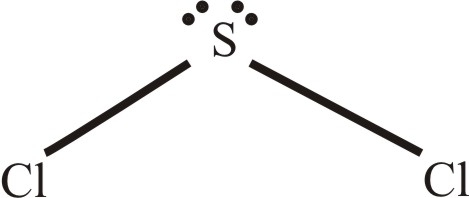
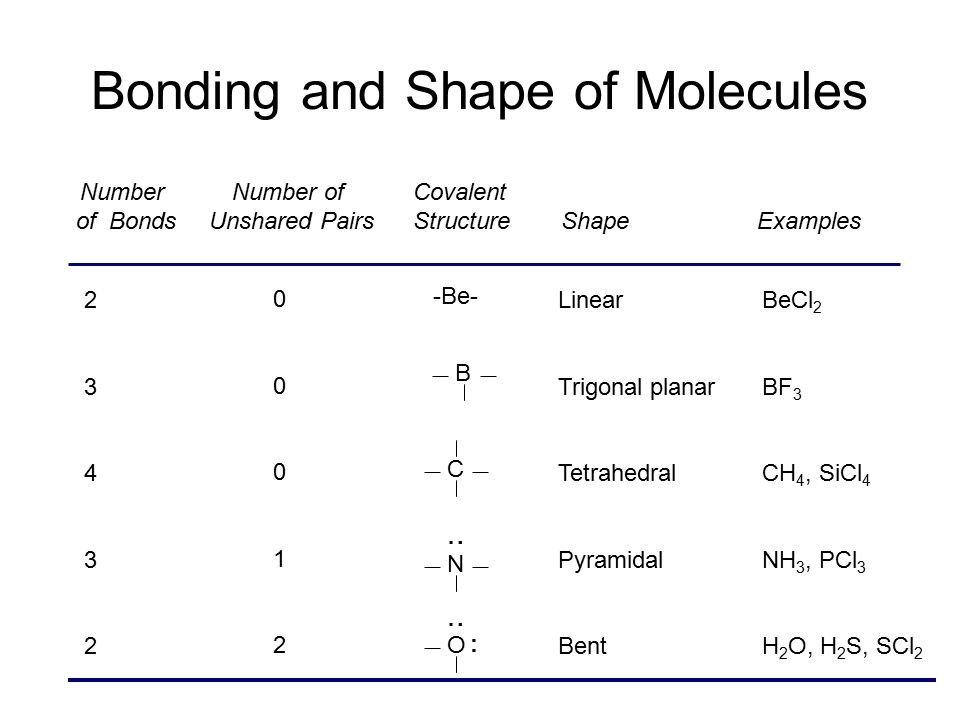
Scl2 lewis structure electron geometry. SCl2 has a bent molecular structure and a tetrahedral electronic shape. Write the Lewis structure for SCl2 and give the electronic and molecular geometry for this molecule. In the same plane the Cl-S-Cl bond forms a 103-degree angle.
The bond angle between. First draw the Lewis dot structure. First of all find out the total number of valence electrons in the C2H4 using the periodic table.
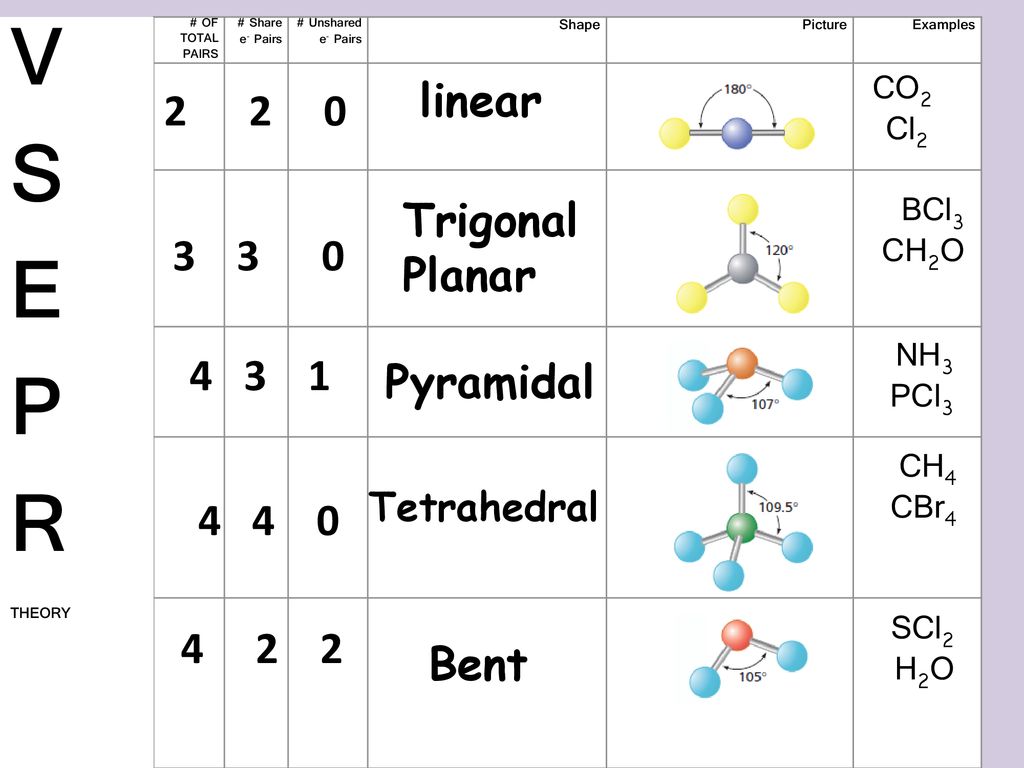
Count how many lone pair and bonded pair electrons it contains. Based on the given Lewis structure there are four electron pairs around the sulfur atom of eqrmSCl_2 eq. Is SCl2 molecule polar or nonpolar.
Well put two between the atoms to form chemical bonds. Just remember all diatomic molecule or any molecule that have only two atoms there geometry or shape will be linear. The sulfur atom here has two bonding pairs shown as horizontal lines and two lone pairs shown as two dots for each pair.
The Lewis Structure of SCl2 sulfur dichloride has one sulfur single-bonded two each of two chlorine atoms. The geometry of the CH3I molecule can then be predicted using the Valence Shell Electron Pair Repulsion Theory VSEPR Theory which states that molecules will choose the CH3I geometrical shape in which the. Draw the Lewis structure of the given molecule.
Note that Sulfur is the least electronegative atom in the SCl2 Lewis structure and is therefore placed in the center. Select all that apply. Draw the Lewis structure for SCl 2 and use it to identify the correct statements that follow.
In SCl2 the sulfur is in group 6 or 16 in the periodic table and has six valence electrons. Hey folks this is me Priyanka writer at Geometry of Molecules where I want to make Chemistry easy to learn and quick to under. A step-by-step explanation of how to draw the SCl2 Lewis Structure Sulfur Dichloride.
The molecule is nonpolar. An explanation of the molecular geometry for the SCl2 ion Sulfur dichloride including a description of the SCl2 bond angles. To draw the SCl2 Lewis structure follow the below instructions.
Because the core central atom sulfur has two S-Cl bonds with the surrounding two chlorine atoms. These electron pairs are two bonding pairs of S-Cl and two lone pairs. It has bond angles of 103.
In the Lewis structure of SCl2 the use of a double bond is necessary. For the SCl2 Lewis structure use the periodic table to find the t. The electron geometry for the.
Sulfur is the central atom in the Lewis structure of SCl2 has a steric number equal to 4 hence electron geometry of SCl2 is tetrahedral. The central atom electron geometry is tetrahedral. Some easy steps to determine the geometry of any molecule.
The structure of thionyl chloride showing the trigonal pyramidal geometry is shown below. Electron geometry which is determined by the steric number will be tetrahedral while molecular geometry which is determined by the coordination number will be bent. The CH3I Lewis structure is a diagram that illustrates the number of valence electrons and bond electron pairs in the CH3I molecule.
Once we know how many valence electrons there are in SCl2 we can distribute them around the central atom with the goal of filling the outer shells of each atom. According to Lewis structure SOCl 2 has three atoms that are attached to central Sulfur that include two Chloride and Oxygen. Upload Choose a File Get more help from Chegg Get 11 help now from expert Chemistry tutors.
As there are four molecules of Chlorine we will calculate the number of valence electrons accordingly. The electron geometry for Cl2 is also linear. The structure has one lone pair of electrons on the central atom.
The electron geometry of SCl2 is determined by the steric number which is equal to the number of sigma bonds present around the atom the number of lone pairs on the atoms. SCl2 has a V-shaped bent molecular geometry and water like electron geometry according to the VSEPR theory. Due to the presence of 4 electron domains and its steric number being 4 the hybridization of SCl2 is given by sp3.
Chemistry QA Library Draw the Lewis electron dot structure for SCl2 and discuss its molecular geometry. Is SCl2 molecule polar. Notice that SCl2 has a molecualr geometry that is very similar to waters the only differences being the smaller bond angle water has a bond angle of 10445 and the longer bond lenght water has a bond lenght of 9584 pm.
The molecular geometry shape is linear.

What Is The Molecular Geometry Of Scl2 Enter The Molecular Clutch Prep
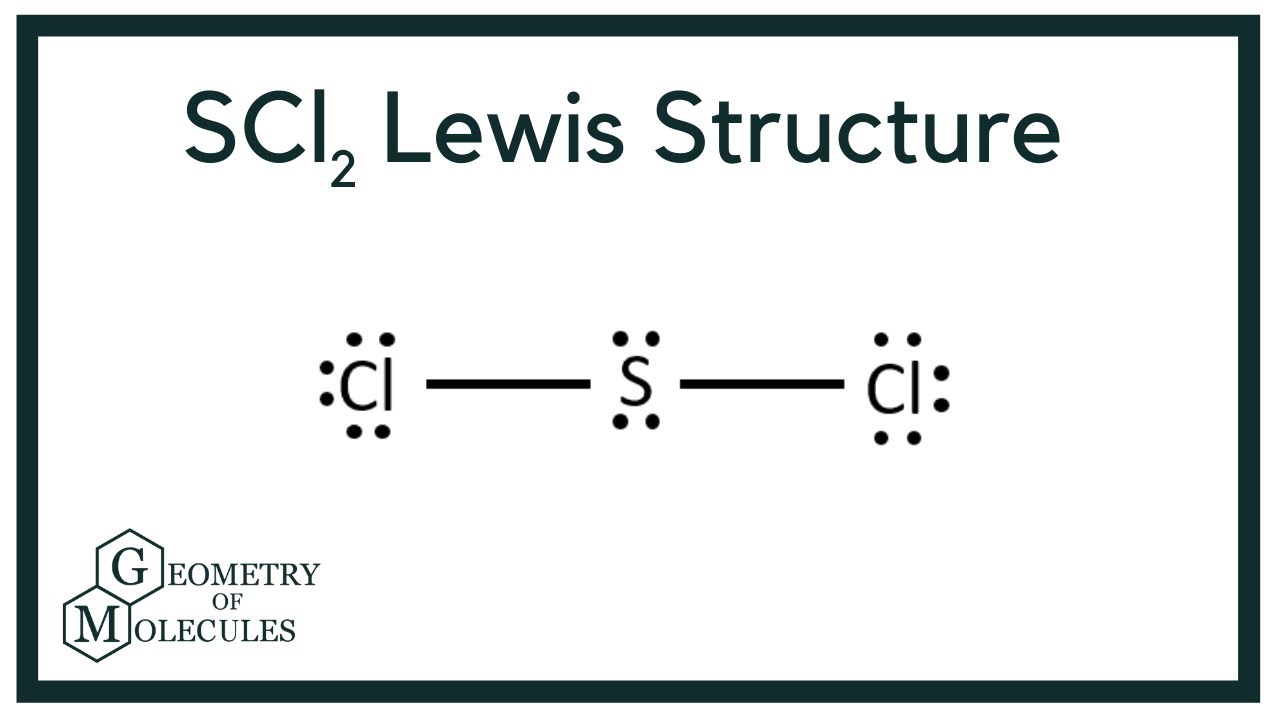
Scl2 Lewis Structure Sulfur Dichloride Youtube

Is Scl2 Polar Or Non Polar Sulfur Dichloride Youtube
Bond Angle Of Scl2 Lewis Structures
Scl2 Lewis Structure Molecular Structure Hybridization Bond Angle And Shape

What Is The Electron Pair Geometry And Mol Clutch Prep

Is Scl2 Polar Or Nonpolar All About Scl2 Polarity

3 6 Predicting The Geometry Of Molecules Chemistry Libretexts

The Lewis Diagram For Scl2 The Electron P Clutch Prep
What Is The Electron Pair Geometry Of Scl2

Solved Predict The Geometry Of Sulfur Dichloride Scl2 And The H Chegg Com
Molecular Geometry And Bonding Theories Ppt Video Online Download

Scl2 Molecular Geometry Sulfur Dichloride Youtube

What Is The Molecular Geometry Of Scl2 Enter The Molecular Clutch Prep

Scl2 Lewis Structure Molecular Geometry
Scl2 Sulfur Dichloride Molecular Geometry Bond Angles Electron Geometry Youtube

Is Scl2 Polar Or Nonpolar Techiescientist

Why Is Scl2 Polar I Have The Lewis Structure And From There I Can See That It Has Polar Bonds Because Of The Cl So Both S Cl Bonds Are Polar Bonds