What Is The Bond Angle Of Xef4
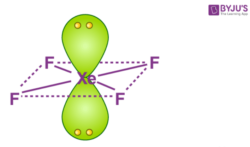
XeF 4 consists of two lone pair electrons. The hybridization in Xenon is sp 3 d 2 because there is a migration of two electrons of p to d orbital which results in the formation of sigma bond with F.
What Will Be The Geometry And Hybridization For Xef4 Chemistry For Neet
Xef4 shape and xef4 bond angle If you aluminate the loan pair then it gives square planner structure.

What is the bond angle of xef4. It is an interhalogen compound. Part B What is the value of the smallest bond angle in XeF4. 1095 Does BF3 have banana bond.
4 bonds and 0 lone pairs. Hybridization- What are the approximate bond angles in this substance. The central atom xenon forms a single bond to four fluorine atoms.
And if I want to find my bond angle in here I know that those three angles have to add up to equal 180 degrees since theyre all in the same plane here. Enter the smallest bond angle of the molecule. What is the bond angle of bf3.
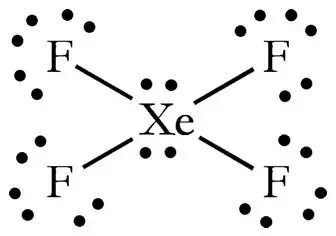
Use lewis structure guidelinesto draw the lewis structure of XeF 4. 120 What is the bond angle of CCl4. XeF4_hybridization XeF4_lewis_structure sp3d2_hybridizationThe structureshapemolecular geometry of Xenon tetrafluoride - XeF4 sp3d2 hybridization of XeF.
Hybridization What are the approximate bond angles in this substance. XeF4 is non polar. The smallest F-Xe-F bond angle in XeF4 is a.
What is the electron geometry of H2O. The bond angles are 90 or 180. So it converted octahedral to square planner.
Loan pair is repulsion. Since there are 4 electron groups around carbon the. View Available Hints degrees Submit.
BF3 has trigonal plannar structure all the three BF bonds lie in plane and thus p-orbitals of boron and fluorine become parallel. This molecule has regions of high electron density that consist of two single bonds and one double bond. And bond angle is 90 each.
Show transcribed image text Expert Answer. What are the bond angles in bcl3. XeF4 Bond angles The bond angles of F-Xe-F are 90 degrees and lone pairs have angles of 180 degrees.
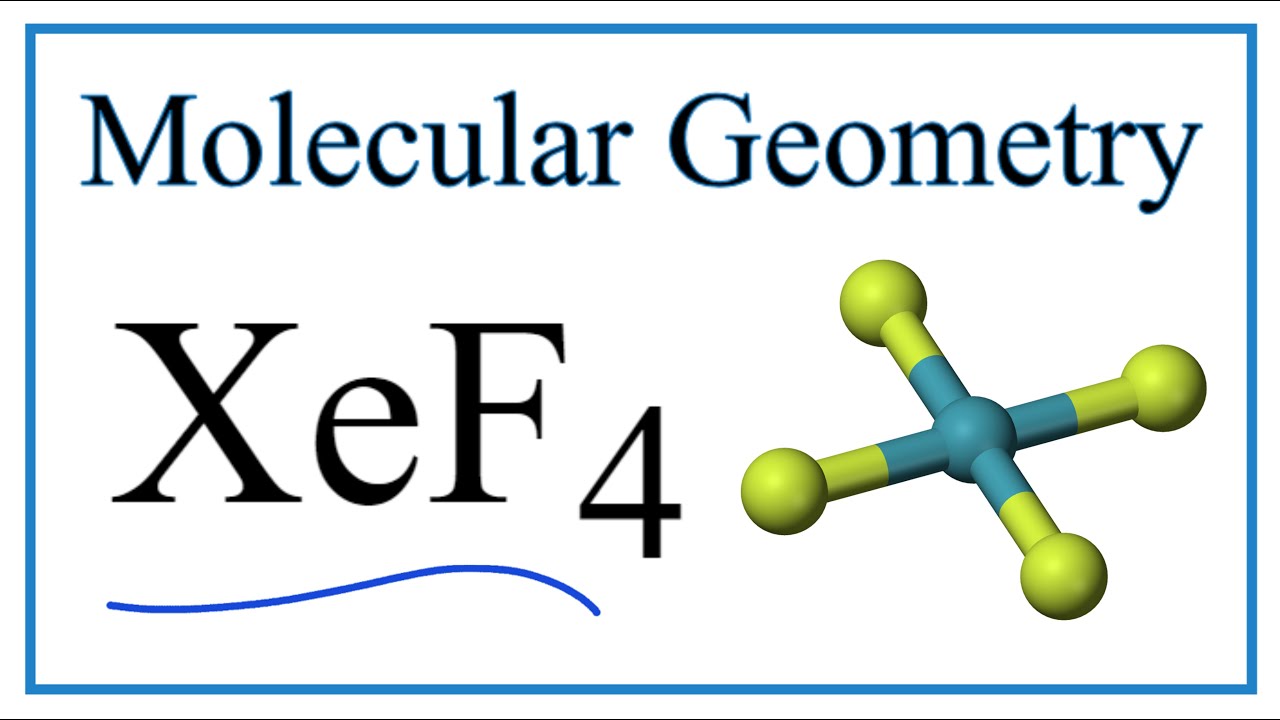
What is the value of the bond angles in ccl4 enter the bond angle of the molecule. The molecular shape of xenon tetrafluoride is square planar. Apply VSEPR notation A X E ANumber of central atoms D 90 and 180.
XeF 4 Molecular Geometry And Bond Angles. An explanation of the molecular geometry for the XeF4 ion Xenon tetrafluroide including a description of the XeF4 bond angles. Therefore XeF4 molecular geometry is square planar.
And as there are 4 fluorine atoms bonding there are 4 bond pairs and 2 lone pairs of electrons. See the answer See the answer See the answer done loading. Science Chemistry Orbital hybridisation.
OXeF bond angles are less than 90 due to lonepair repulsion. Hence the overall molecular geometry of XeF4 comes out to be square planar with a bond angle equal to 90 or 180. Hence there are a total of 36 valence electrons in XeF4.
Preparation of xef4 there are two methods is used for the preparation of xenon tetrafluoride. What is the hybridization of the central atom in AsFs. What shape would you expect for XeF4.
What is the bond angle of xef4. This problem has been solved. The electron geometry for th.
There are 4 of these so around the xenon atom there are 84 12 electrons so 6 electron pairs. XeF4 Molecular Geometry And Bond Angles In order to achieve this the lone pairs lie in a perpendicular plane in an octahedral arrangement opposite 180 degree from each other. Bond angles- 03 A.
Carbon is surrounded by 4 electron groups. Now if we follow the VSEPR theory the net. XeF 4 consists of two lone pair electrons.
It has 4 bond pairs and 2 lone pairs hence it has a square planar shape. What is the hybridization of the central atom in XeF4. The basic geometry is trigonal planar with 120 bond angles but we see that the double bond causes slightly larger angles 121 and the angle between the single bonds is slightly smaller 118.
Therefore this is an octahedral shape with 90 degree angles between each bond and lone pair of electrons. XeF4 has an electronic geometry of octahedral making the molecular geometry of Xenon Tetrafluoride square planar. The Lewis structure of CCl4 is.
Xef4 Molecular Geometry Bond Angles Electron Geometry Youtube

Xef4 Molecular Geometry Bond Angles Electron Geometry Youtube
Answer In General Chemistry For Edidiong Michael Inyang 88664

Hybridization Of Xef4 Xenon Tetrafluorid Youtube

Xef4 Xenon Tetrafluoride Sp3d2 Hybridization Structure Shape Bond Angle Lone Pairs Adichemistry Youtube

Xef4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
What Is The Vsepr Structure Of Xef4 Quora
Why Is The Shape Of Xef4 Not Tetrahedral Quora

Xef4 Lewis Structure And Molecular Geometry Youtube

Xef4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
Hybridization Of Xef4 Hybridization Of Xe In Xenon Tetrafluoride
How Can The Lewis Structure For Xef4 Be Determined Quora

Xef4 Lewis Structure And Molecular Geometry Youtube
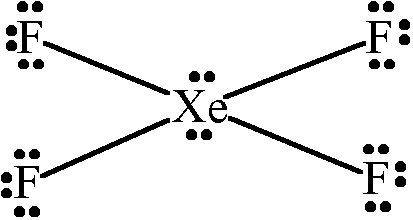
Which Of The Following Statments Concerning Xef4 Is Chegg Com
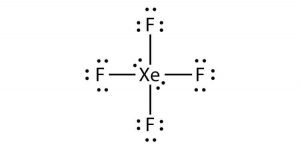
Xef4 Xenon Tetrafluoride Molecular Geometry Lewis Structure And Polarity Geometry Of Molecules
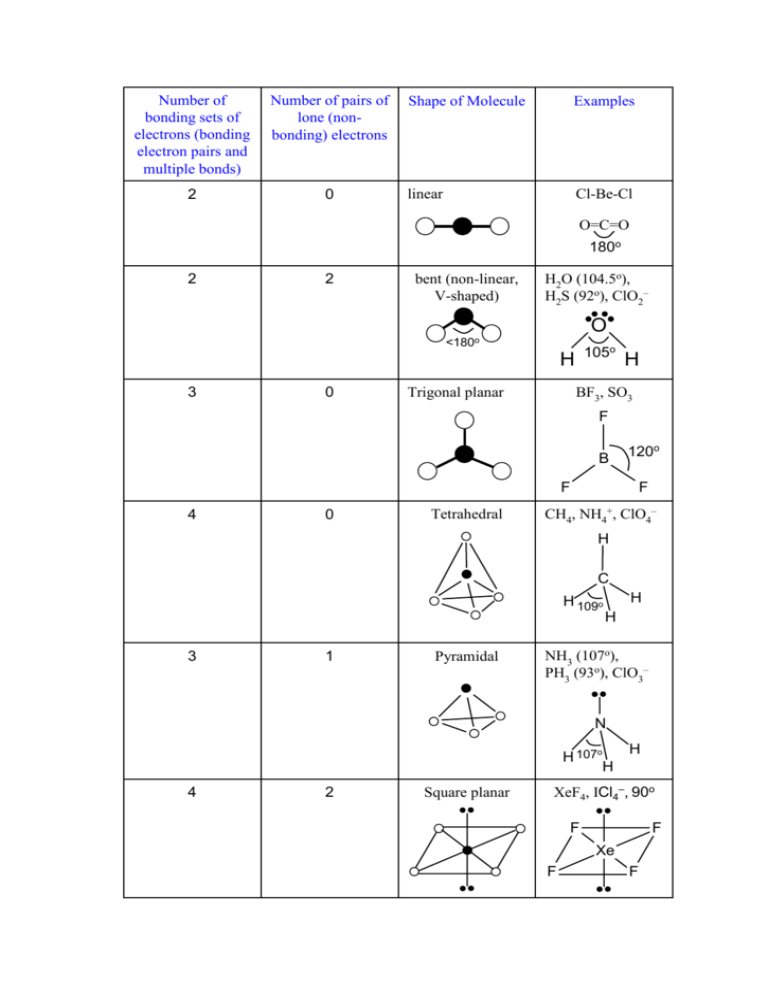
Xef4 Icl4 90o Square Planar 2 4 Nh 107o Ph 93o Clo
What Is The Vsepr Structure Of Xef4 Quora
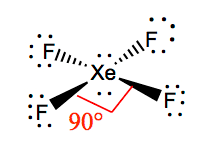
Xef4 Xenon Tetrafluoride Molecular Geometry Lewis Structure And Polarity Geometry Of Molecules
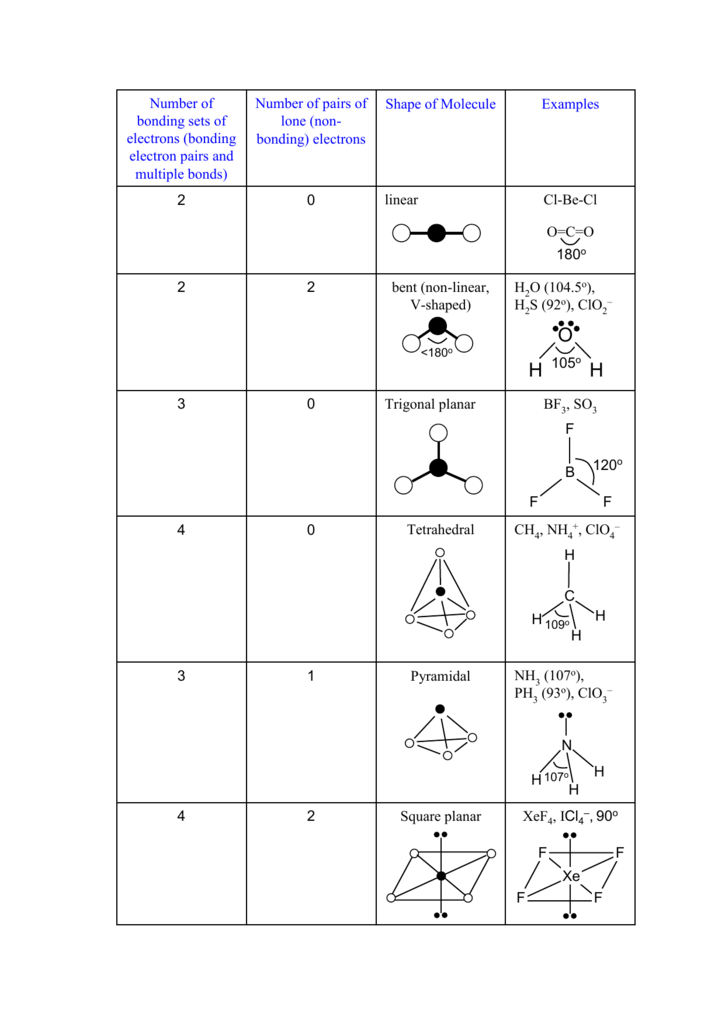
Xef4 Icl4 90o Square Planar 2 4 Nh 107o Ph 93o Clo