H2so4 Lewis Acid Or Base
A Lewis acid is defined as an electron-pair acceptor whereas a Lewis base is an electron-pair donor. Under this definition we need not define an acid as a compound that is capable of donating a proton because under the Lewis definition H itself is the Lewis acid.

What Is Difference Between Hydrochloric Acid And Sulfuric Acid Quora
Aluminum chloride lewis Acid.

H2so4 lewis acid or base. H2SO4 is strong Acid. 24 10 1. Identify the acid and the base in each Lewis acidbase reaction.
Pure water and acid neutral aqueous solutions have equal amounts of H and OH-. H2SO4 is not amphoteric because it can not act as a base. In other words a Lewis acid is an electron-pair acceptor.
Lewis acids and bases Brönstead acids can be thought of as electron deficient ions. Sulfuric acid can supply up to 2 H ions per molecule and H ions are Lewis Acids. Weitergeleitet von H2SO4 Schwefelsäure ist eine chemische Verbindung des Schwefels mit der Summenformel H 2 SO 4.
Brönstead acids and bases contain H and OH-HCl H Cl- acid NaOH Na OH-base. CH 3 2 O BF 3 CH 3 2 OBF 3. This is because with no electrons H can accept an electron pair.
NO 3-Nitrate ion-----Hydronium ion. Linked to so3 lewis acid or base Acid reflux disorder dilemma is usually a condition of your digestive method of human anatomy. For example a coordinate covalent bond occurs when a water molecule.
Sie ist eine farblose ölige sehr viskose und hygroskopische Flüssigkeit. 13 10 6. 32 10 9.
ClO 4 -Perchlorate ion. CH 3 2 O. Iii Lewis defined the acid as an electron pair acceptor and the base as an electron-pair donor.
If it were able to act as a base then you should get H3SO4 as product. Water is acid and base simultaneously H 2O H OH-. H2SO4 is sulfuric acid a diprotic strong acid not a base.
A Lewis acid is any substance such as the H ion that can accept a pair of nonbonding electrons. For example NH3 is a Lewis base because it can donate its lone pair of electrons. H 2 SO 4.
H2So4 is a lewis acid because it is a bronsted acid. But instead of Lewis acid- base theory these type of compounds are classified as Bronsted-Lowry acidsbases where an acid is considered a substance that is a proton donor H and a base is a proton acceptor. List molecules Acid and Base.
A coordinate covalent bond or dative bond occurs when one of the atoms in the bond provides both bonding electrons. If you want to quickly find the word you want to search use Ctrl F then type the word you want to search. Is this acid or base H2SO4.
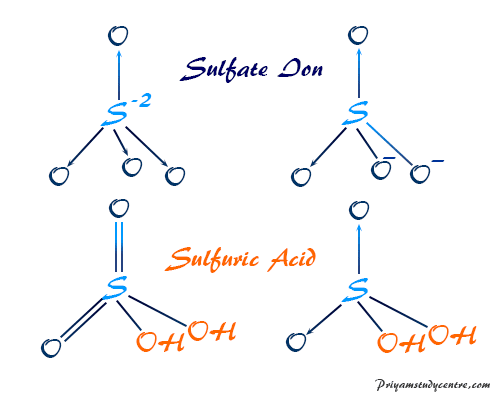
H 2 O SO 3 H 2 SO 4. When a Brønsted-Lowry acid loses a prot. Lewis proposed a generalized definition of acid-base behavior in which acids and bases are identified by their ability to accept or to donate a pair of electrons and form a coordinate covalent bond.
Schwefelsäure ist eine der stärksten Säuren und wirkt stark ätzend. A Lewis base then is any species that has a filled orbital containing an electron pair which is not involved in bonding but may form a dative bond with a Lewis acid to form a Lewis adduct. A Lewis base is any substance such as the OH- ion that can donate a pair of nonbonding electrons.
H2SO4 is the chemical formula for Sulphuric acid which is a strong acid. Acid reflux disorder can manifest to youthful and previous no matter the age. A Lewis base is therefore an electron-pair donor.
We can rationalize the observed reactivity on the basis of all three definitions. Ill tell you the Acid or Base list below. 10 10 3.
Trimethylborane is a Lewis acid. 2 H 2 SO 4 H 3 SO 4 HSO 4. Two Types of Acids and Bases.
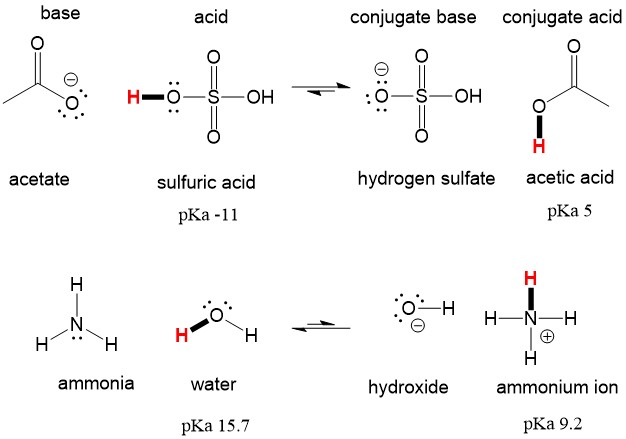
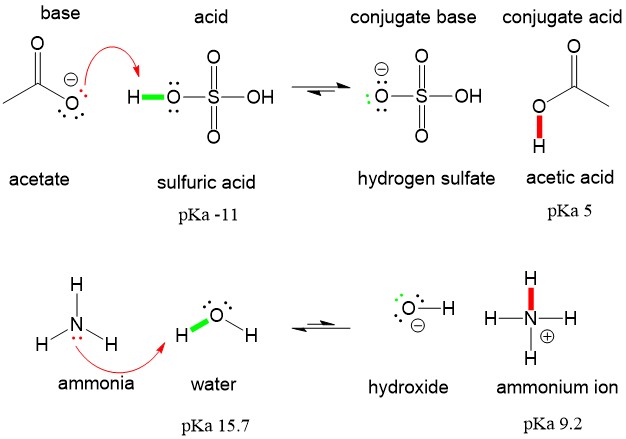
In the BrønstedLowry definition of acids and bases an acid is a proton H donor and a base is a proton acceptor. H 2SO4 N H 3 N H 4 H SO 4. Acid Base Conjugate acid and conjugate base.
A Lewis acid is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. ALL bronsted acids are lewis acids but not the other way around. Is H2SO4 Acid or Base.
H2SO4 SULFURIC ACID is strong acid What is an acid base neutral. The answer is D. 10 10 9.
Frequent episodes of acid refluxes can damage the lifestyle of the man or woman by supplying him sleepless nights. HSO 4-Hydrogen sulfate ion. Similarly it may be said that sulfate ion SO4-2 acts as Lewis base when hydrogen ions H quickly accept an electron pair from sulfate ion to form H2SO4 sulfuric acid.
One use of nonaqueous acid-base systems is to examine the relative strengths of the strong acids and bases whose strengths are leveled by the fact that they are all totally converted into H 3 O or OH ions in water.
Reviewing Acid Base Definitions Organic Chemistry Help

New Page 2 731x498 Jpeg Organic Chemistry Organic Chemistry Reactions Chemistry
Sulfuric Acid H2so4 Structure Production Properties Uses

Deciding Sn1 Sn2 E1 E2 1 The Substrate Master Organic Chemistry Organic Chemistry Teaching Chemistry Organic Chemistry Reactions

A Reaction Map Pdf For Benzene And Aromatic Compounds Organic Chemistry Reactions Organic Reactions Reactions

Unit 2 Organic Chemistry Organicheskaya Himiya Himiya

Draw And Explain The Lewis Structure For Sulfuric Acid Study Com

H2so4 Lewis Structure Sulfuric Acid Youtube

Dissolution Mechanism Of Cellulose In 72 Wt Sulfuric Acid Based On Download Scientific Diagram
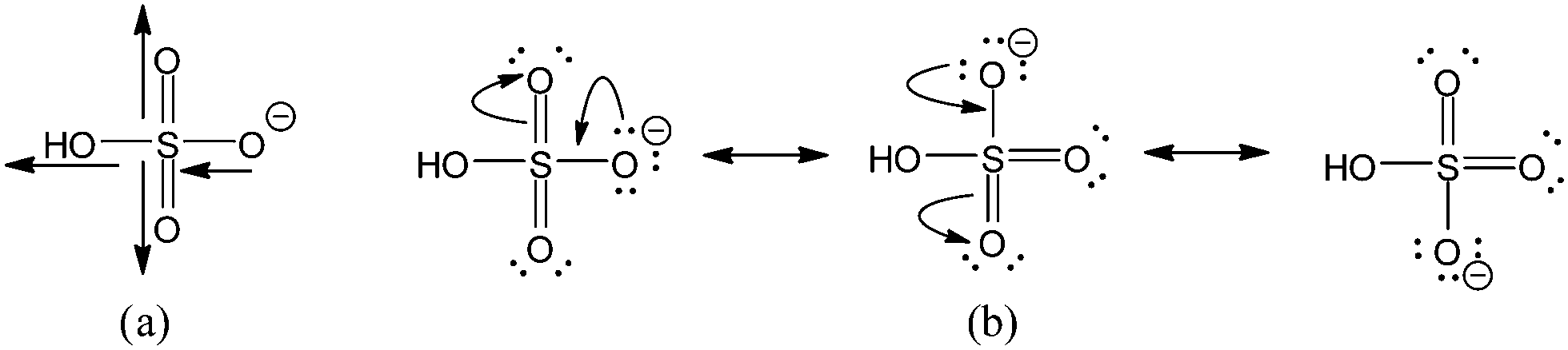
Why Is Sulfuric Acid A Much Stronger Acid Than Ethanol Determination Of The Contributions By Inductive Field Effects And Electron Delocalization Effe Physical Chemistry Chemical Physics Rsc Publishing Doi 10 1039 C4cp04110k
Sulfuric Acid H2o4s Chemspider

Is H2so4 An Acid Or Base Strong Vs Weak Sulfuric Acid
Reviewing Acid Base Definitions Organic Chemistry Help

Non Aqueous Solvents Nh3 Hf H2so4 N2o4 Pocl3 Socl2 Brf3 In 2021 Solvents Chemistry Agno

Tetryonics 56 04 Common Acids Are Formed Where Protons Bind To Chemical Elements And Compounds To Create Incomplete Atomic Orbitals And Charged Cations



