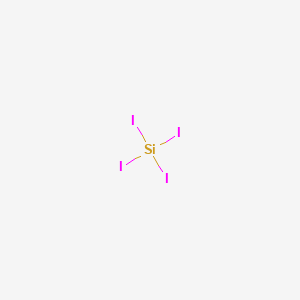
Sii4 Valence Electrons
6 72 20 valence. What is its molecular compound name.
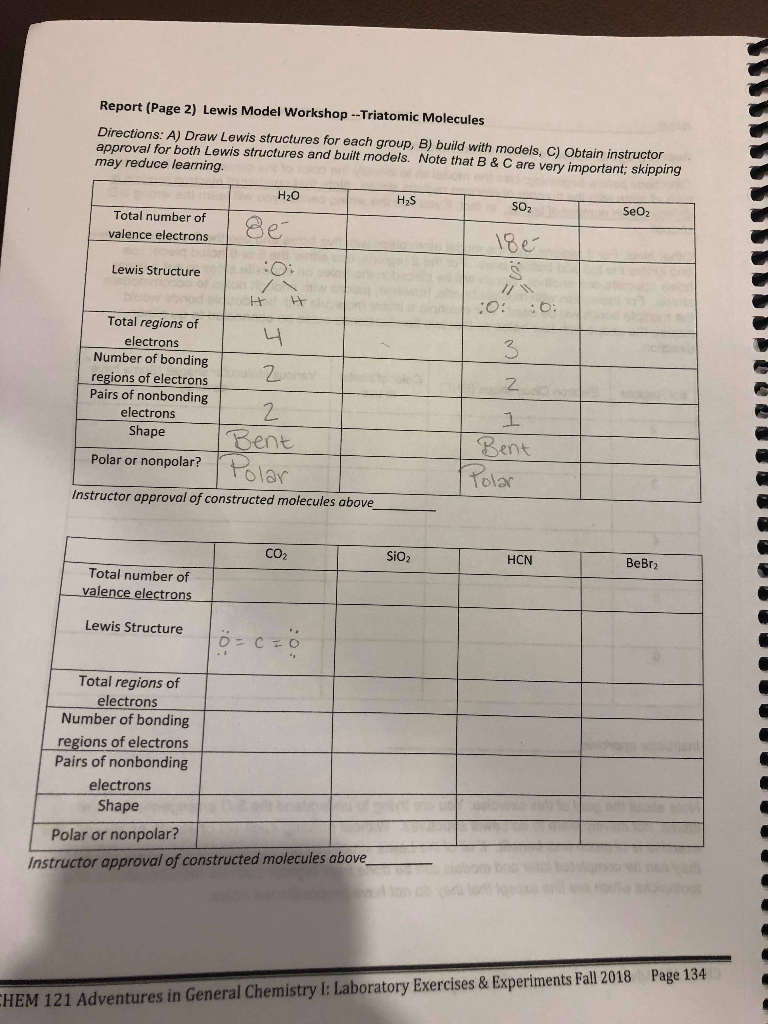
H2s Seo2 Co2 Sio2 Hcn Bebr2 Total Number Of Chegg Com
Were going to do the Lewis structure for SiF4.

Sii4 valence electrons. SiI 4 is a precursor to silicon amides of the formula Si NR 2 4 R alkyl. It is used in smoke screens to make various silicon containing chemicals and in chemical analysis. Sil - use a black ball for silicon when constructing this model Molecular Shape ar On-Polar Total of Valence Electrons.
It has also been of interest in the manufacture and etching of silicon in microelectronics. Any polar bonds in the molecule. Write the formula for each of the following ionic compounds.
So lets start out. We have four of them. Molecules and Compounds Instructions.
Silicon tetrachloride is a colorless fuming liquid with a pungent odor. One of the easiest ways to find valence electrons is by checking out the elements place in the periodic table. Any polar bonds in the molecule.
A pair of electrons occupying an orbital in an atom or molecule and not directly involved in bonding is called as lone pair of that atom. Total valance electrons pairs σ bonds π bonds lone pairs at valence shells. Total valence electrons in OF2 Valence electrons of Oxygen Valence electrons of Fluorine.
In chemistry and physics a valence electron is an electron in the outer shell associated with an atom and that can participate in the formation of a chemical bond if the outer shell is not closed. The common name of SiI4 is tetraiodosilane. On the periodic table Silicon group 4 4 valence electrons.
So 4 plus 4. Valence electrons also determine the electrical conductivity of an element. So lets put two electrons between each of the atoms there to give it a chemical bond.
Yes No Molecular Polarity. The presence of valence electrons can determine the elements chemical properties. Oxygen has six valence electrons in its outer shell.
3-D Model Sketch Lewis Structure show all resonance structures if applicable Bond Angles Molecular Shape. It is corrosive to metals and tissue in the presence of moisture. Hydrogen group 1 1 valence electron but we have four of them.
How many of these valence electrons take part in covalent bonds. Sodium chloride Zinc chloride Ammonium chloride Potassium hydroxide Calcium Nitrate. Find the mass ratios and atomic ratios of the following compounds.
You can find lone pair of any atom by knowing its valency. On the periodic table Silicon is in group 4 sometimes called 14 so its got 4 valence electrons. HCN Total of Valence Electrons.
Fluorine group 7--7 valence electrons but we have 4 of those so were going to multiply that by 4. Lewis Structure show all resonance structures if applicable 3-D. Yes No Any polar bonds in the molecule.
How many valence electrons does SulfurS have. SiI4 was proposed as a potential precursor for the deposition of Si-based films by CVD. The valence electrons of an element.
3-D Model Sketch Bond Angles. Sil4 - use a black ball for silicon when constructing this model Total of Valence Electrons. Put the Si in the center Hydrogens always go on the outside.
So we have 14 valence electrons from Fluorine atoms. SiI4 has a simple structure. 8 total valence electrons.
For NO 2 Total pairs of electrons are 8 and one electron exists as unpaired electron because 17 cannot be divided exactly by 2. CI2O7 SbF5 NH3 BaI2 S2Cl2 OsO4 Instructions. Total valence electrons pairs.
3-D Model Sketch Bond Angles Lewis Structure show all resonance structures if applicable Molecular Shape. Silicon tetraiodide is the chemical compound with the formula Si I 4. Chloroform CHCl3 was one of the first anesthetics used in medicine.
Determination of Valence Electrons. Depending on in this nature of elements can be a metal non-metal or a metalloid. And that is 4 plus 28 is 32.
SiI4 is advantageous as it is a simple Si-contaning precursor which can be used in thermally or UV-assisted CVD deposition processes. Nitrogen has 5 electrons in its valence s. Total of Valence Electrons.
The chloroform molecule contains 26 valence electrons in total. Total electron pairs are determined by dividing the number total valence electrons by two. In a single covalent bond both atoms in the bond contribute one valence electron in order to form a shared pair.
So we have 32 valence electrons to work with. Yes No Molecular Polarity. It is a tetrahedral molecule with Si-I bond lengths of 2432 5 Å.
It is decomposed by water to hydrochloric acid with evolution of heat. Each fluorine atom has seven valence electrons but as there are two Fluorine atoms we will multiply the number by 2.

Chapter 8 Chemical Bonding Study Guide Flashcards Quizlet

Sif4 Lewis Structure How To Draw The Dot Structure For Sif4 Youtube

Solution Chem 10 Smc Lewis Structures And Molecular Shapes Worksheet Studypool
Silicon Tetraiodide Sii4 Pubchem

Ch 12 Chemical Bonding Ppt Video Online Download

Lewis Structures And Molecular Shapes 3 D Model Chegg Com

Reportpage3 Wk2lab Damasor Pdf N02 Pf3 Sii4 Crude Sketch N I I F P F A 0 0 I 1 5 1 Calculations Of Valence Electrons Of Bonds Etc 5 6 2 1 5 7 3 4 7 4 Course Hero

Hello I Need To Fill Out These Boxes For Many Chegg Com
3 Sil4 Use A Black Ball For Silicon When Chegg Com
3 Sil4 Use A Black Ball For Silicon When Chegg Com