So3 Lewis Structure Shared Bonding Electrons
The actual structure of SO 3 has a double bond to one oxygen and dative bonds to two other oxygens. A double bond between two atoms A and B Multiple Choice has a lower bond energy than a single bond between the same two atoms.

Calculating So3 Formal Charges Calculating Formal Charges For So3 Youtube
Now have a look of Lewis Structure again.
So3 lewis structure shared bonding electrons. Hybridization of SO 3 molecule. Multiple Choice 19 2. Is longer than.
They donate a pair of electrons as well but in this case the pair is shared between two atoms. Shared bonding pairs of electrons. The lewis structure of SO3 is being considered when no electrons will interfere with the synthesis of SO3 however this will change dramatically when electrons are added.
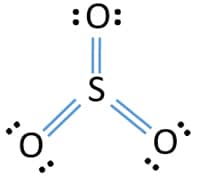
Each oxygen atom has two lone pairs in SO 3 lewis structure. Deduct the electrons in forming the bonds from step 1 while noting that a single bond is made up two electrons. Because we add 2 electrons we have to account for that in the determining factor valence electrons.
The charge an atom would have if all the electrons in bonds were shared equally. What is the Lewis structure of methanethiol CH3SH. S-O bond is not a single bond.
This not only covers double bonds but also triple bonds alkynes as well as aromatics and even enols and enolates in Org 2. Calculate the total valence electrons in BF 3 molecule. SO 3 has 24 valence electrons.
Unshared lone nonbonding pairs of electrons. Lewis dot structure of SO 3 2-Alternatively a dot method can be used to draw the lewis structure of BF 3. The best Lewis structure-is the one with the.
How many shared pairs are in this molecule. Draw the Lewis structure of SO3. Consider the structure shown.
The Lewis structure for SO 3 is requires you to place more than 8 valence electrons on Sulfur S. Exceptions to the Octet Rule. Select the single best answer.
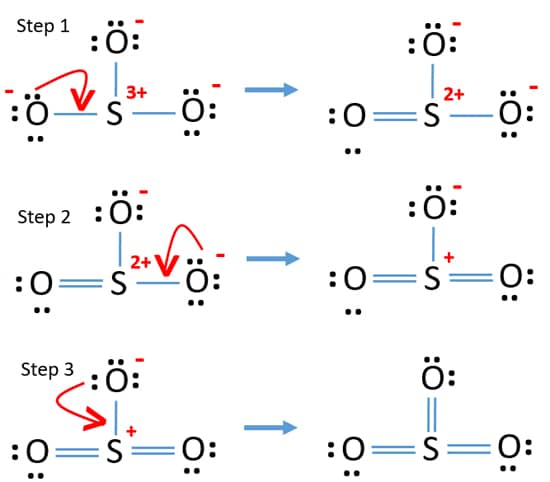
Total outermost valence shell electrons available for SO3 lewis structure dot structure 6 63 24 valence electrons in SO3. Dative bonds are formed when the bonding electrons are both donated from one atom. How to draw the Lewis Structure of SO3 sulfur trioxide - with explanationSulfur is an exception to the octet rule - it can handle up to 12 electronsCheck.
Unshared lone nonbonding electrons. This gives S its octet using 8 of the valence electrons. Determine the number of shared and unshared pairs of electrons in the structure.
Proceed to the next steps to learn how higher bond orders are possible in Lewis dot structures. Youll want to calculate the formal charges on each atom to make sure you have the best Lewis structure for SO 3. A bond order of 3 would represent the sharing of 3 electron pairs or 6 electrons total.
Each of the bonds above currently have a bond order of 1. 6 3 x 6 24. The diagram is drawn showing dots of valence electrons around the symbol of both sulfur and oxygen atoms with lines predicting bond formation.
Determine the number of shared and unshared electrons in the structure. Remember Sulfur is in Period 3 and can hold more than 8 valence electrons. Calculation of total valence electron of SO3 molecule.
The central S is bonded to each of the O atoms. A step-by-step explanation of how to draw the SO3 Lewis Dot Structure Sulfur trioxideFor the SO3 structure use the periodic table to find the total number. Total24226 Put sulfur in the center and three oxygen atoms on the sides.
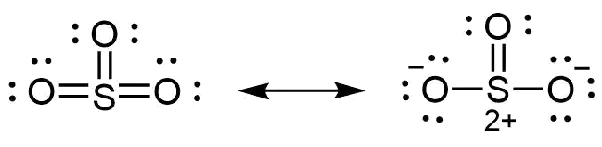
Ions or molecules with an odd number of electrons. One Lewis structure cannot accurately depict a molecule We use multiple structures to describe the molecule. The bond order of sulfur and oxygen atom is not one.
Consists of two electrons shared between A and B. Lewis Structure of SO3. Draw each atoms six valence electrons in pairs of two electrons.
SO3 has a total of 24 valence electrons. In this case the dative bonds are formed when oxygen bonds to the lone pair electrons formed during sp 2 hybridization. Here is the long answer.
Put a pair of electrons connecting the side atom with central atomPut remaining electrons on the side atomsMake sure each side atom get 8 electrons to. Sulfur brings 6 and oxygen brings 3 each. When we draw it firstly we get the three structures at the top.
The sulfur trioxide is a tetra atomic chemical molecule where both the sulfur and three oxygen molecules bond with an equal number of valence electrons. But there is no lone pair on sulfur atom in SO 3 lewis structure as lewis structure of SO 2. Here sulfur in the center because of its lowest electron capability and three oxygen around it.
π bonds can also be thought of as nucleophiles. There are three double bonds around sulfur atom with oxygen atoms in SO molecule. You might think youve got the correct Lewis structure for SO 3 at first.
Lewis structure of SO3. In the dot structure one of the O atoms is double bonded to the S while the others have a single bond. Predict the polarity of SO3.
What Is The Difference Between The Lewis Structure Of So3 Vs So3 2 Quora

So3 Molecular Geometry Lewis Structure And Polarity Explained

How To Determine The Lewis Dot Structure Of So3 Quora
So3 Molecular Geometry Lewis Structure And Polarity Explained
Lewis Structure Of So3 Sulfur Trioxide Youtube
Sulfur Trioxide So3 Lewis Structure Hybridization Drawing Steps

So3 Lewis Structure Molecular Geometry And Hybridization Techiescientist

How To Determine The Lewis Dot Structure Of So3 Quora

So3 Lewis Structure How To Draw The Lewis Structure For So3 Sulfur Trioxide Youtube

Calculating So3 Formal Charges Calculating Formal Charges For So3 Youtube

Calculating So3 Formal Charges Calculating Formal Charges For So3 Youtube
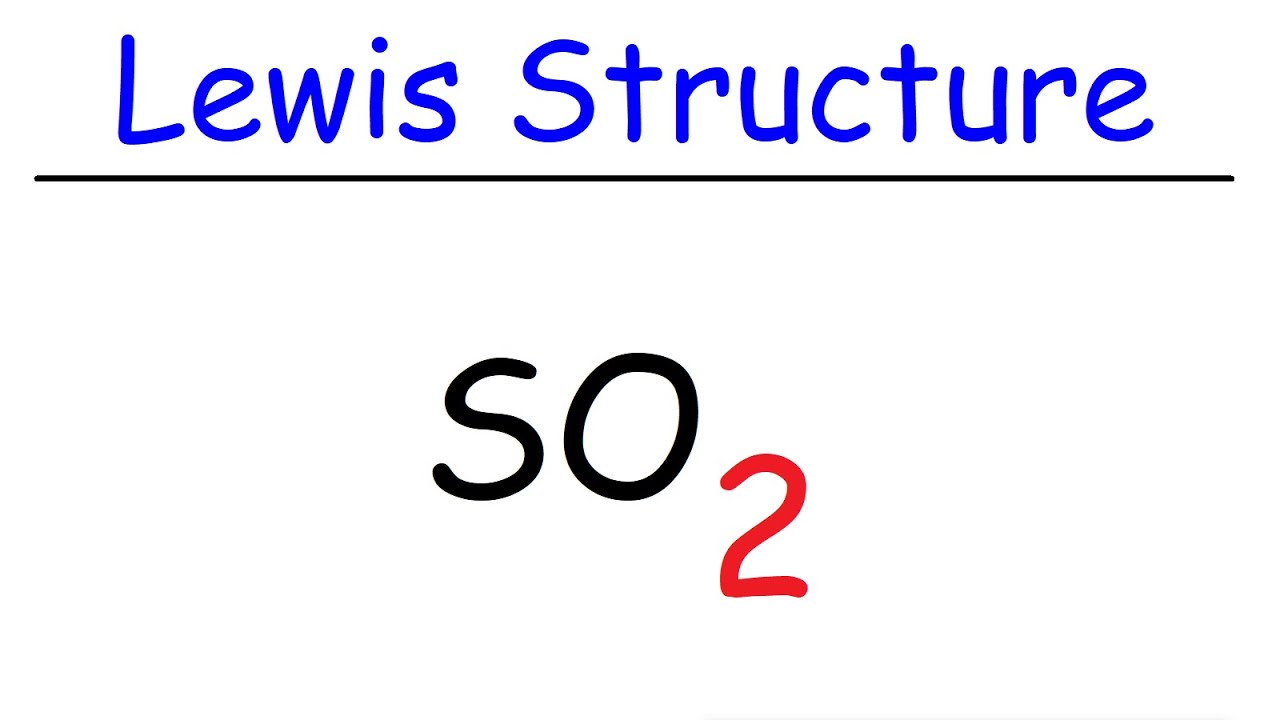
So3 Lewis Structure Sulfur Trioxide Youtube

Sulfur Trioxide So3 Lewis Structure Hybridization Drawing Steps

So3 2 Lewis Structure How To Draw The Dot Structure For So3 2 Lewis Structure Chemical Bonding

So3 2 Lewis Structure How To Draw The Dot Structure For So3 2 Lewis Structure Chemical Bonding

How To Determine The Lewis Dot Structure Of So3 Quora

So3 Lewis Structure Molecular Geometry And Hybridization Techiescientist
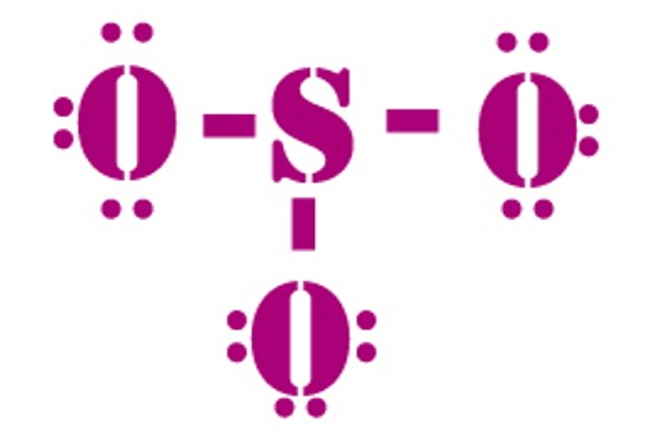
So3 Molecular Geometry Lewis Structure And Polarity Explained
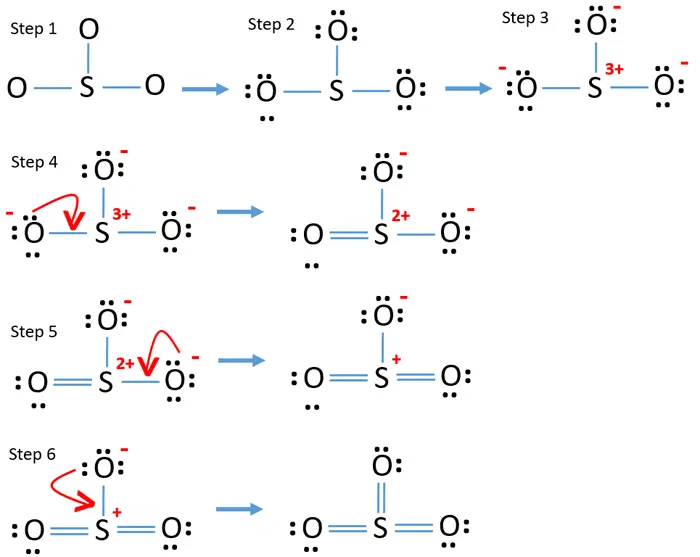
Sulfur Trioxide So3 Lewis Structure Hybridization Drawing Steps