Brf3 Lewis Structure Hybridization
When one atom of bromine reacts with three atoms of fluorine to form BrF3 what hybridization must Br have in bromine trifluoride. Hence the hybridization would be sp3d.
What Is The Hybridization Of Brf3 Quora
Boron trifluoride is the inorganic compound and its formula is BF3.
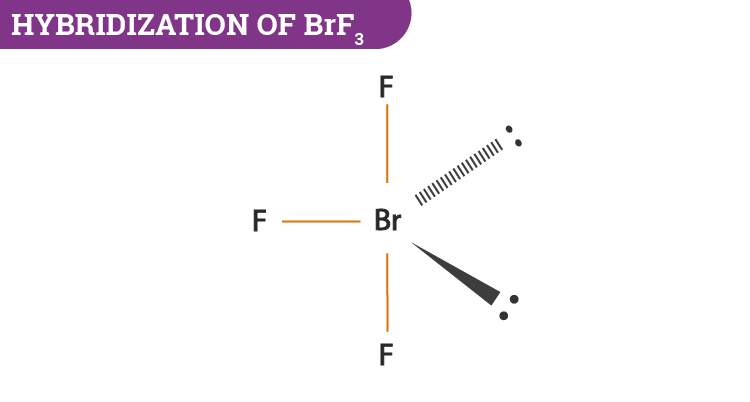
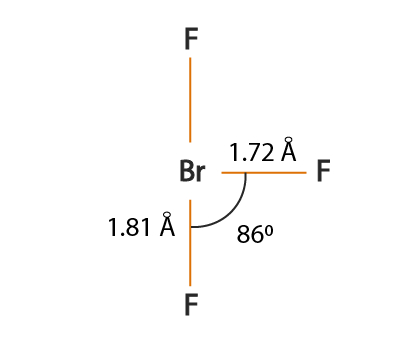
Brf3 lewis structure hybridization. BrF3 has a Bent T shaped structure with Br as a central atom bonded with three F atoms three bond pairs and two lone pairs. BrF3 has a Bent T shaped structure with Br as a central atom bonded with three F atoms three bond pairs and two lone pairs. BrF3 is a polar molecule due to the presence of two pairs of lone pair electrons.
It contains two lone pairs and three BrF covalent bonds after bond formation. There are a total of 28 valence electrons for the BrF 3 Lewis structure. After the bond formation it will further have two lone pairs and 3 BrF covalent bonds bonding pairs.
BF3 has a maximum of 24 valence electrons which we have to place around the central atom. BrF3 Lewis Structure Molecular Geometry Hybridization and MO Diagram BrF3 known as Bromine Trifluoride is a fuming liquid consisting of inter-halogen combinations and bearing a pungent smell. N2H2 and BrF3 Draw the Lewis structure Label the hybridization of each central atom Draw a valence bond diagram for the molecule being sure to label each of the orbitals.
The structure of the BF3 molecule based on hybridization is given below. Such compounds are known as inorganic compounds as they are not the organic ones because of lacking Carbon. Hence the hybridization would be sp3d.
A sp3d2 B sp3d C sp3 D sp2 E sp. As the hybridization value or the electron pair is equal to 5 it gives rise to sp3d hybrid orbitals. You may have heard about the chemical compound that lacks C-H bonds.
Image will be Uploaded Soon To know about the BF3 Lewis structure we have to calculate the total valence electrons count for the BF3 molecule. Now Bromine can use the d-orbitals for hybridization. There are a total of 28 valence electrons for the BrF 3 Lewis structure.
What is the Hybridization of Bromine Trifluoride. Now bromine can use the d-orbitals for hybridization. The electron pairs hybridization value is equal to 5 resulting in sp 3 d hybrid orbitals.
XeF2 structure features two covalent bonds between one xenon atom and two fluorine atoms. Br and F will form bonds and will have two lone pairs and three covalent bonds. The BrF3 has seven electrons in the outermost shell for hybridization.
BF3 Lewis Structure Molecular Geometry Hybridization and Polarity. 84 382 ratings play-rounded-fill. After determining how many valence electrons there are in BrF 3 place them around the central atom to complete the octets.
For the BrF 3 Lewis structure calculate the total number of valence electrons for the BrF 3 molecule. When a bromine atom reacts with a single fluorine atom to form a BrF molecule a single sigma bond is formed. Draw the Lewis structure of BrF3 and determine the bond angle between an equatorial F atom and an axial F atom 90º 90º 120º 120 1095º.
Bromine PentafluorideBrF5 is a polar molecule because the molecular geometry of BrF5 is square pyramidal with an asymmetric charge distribution and with a bong angle of 90The molecule has a central bromine atom surrounded by five fluorides and a pair of electrons. After the bond formation it will further have 2 lone pairs and 3 BrF covalent bonds. What is the hybridization on the Br atom.
QUESTION 22 Draw the Lewis structure for BrF4. Asked Jun 20 2017 in Chemistry by Eaesman. Is BrF5 Polar or Nonpolar.
A sp3d2 B sp3d C sp3 D sp2 E sp. In its outermost shell BrF3 has seven electrons. A step-by-step explanation of how to draw the BrF3 Lewis Dot Structure Boron trifluoride For the BrF3 structure use the periodic table to find the total n.
QUESTION 22 Draw the Lewis structure for BrF4. The Br-F bonds and the hybridization value answer is 5. Having a straw ie colorless to yellow appearance this chemical compound has several applications but also comes with a number of limitations and hazard issues.
In identifying the hybridization of bromine trifluoride take the bromine atom and check its electron configuration and D-Orbitals. 2What is the Lewis structure. As the hybridization value or the electron pair is equal to 5 it gives rise to sp 3 d hybrid orbitals.
Bromine is the least electronegative atom in the BrF 3 Lewis structure and therefore goes at the center of the. BrF3 consists of seven electrons in its outermost shell. Hence its hybridization is sp3d.
What is the hybridization on the Br atom. Both need 1 extra electron to complete their octets. What is the structure of XeF2.
BrF 3 will consist of seven electrons in its outermost shell. Important Points To Remember.
Hybridization Of Brf3 Hybridization Of Br In Bromine Trifluoride
What Is The Hybridization Of Brf3 Quora
Molecular Shape Of Brf3 P Block Elements Chemistry Class 12 Youtube
What Is The Hybridization Of Brf3 Quora
Brf3 Polarity Molecular Geometry Hybridization And Bond Angle Geometry Of Molecules
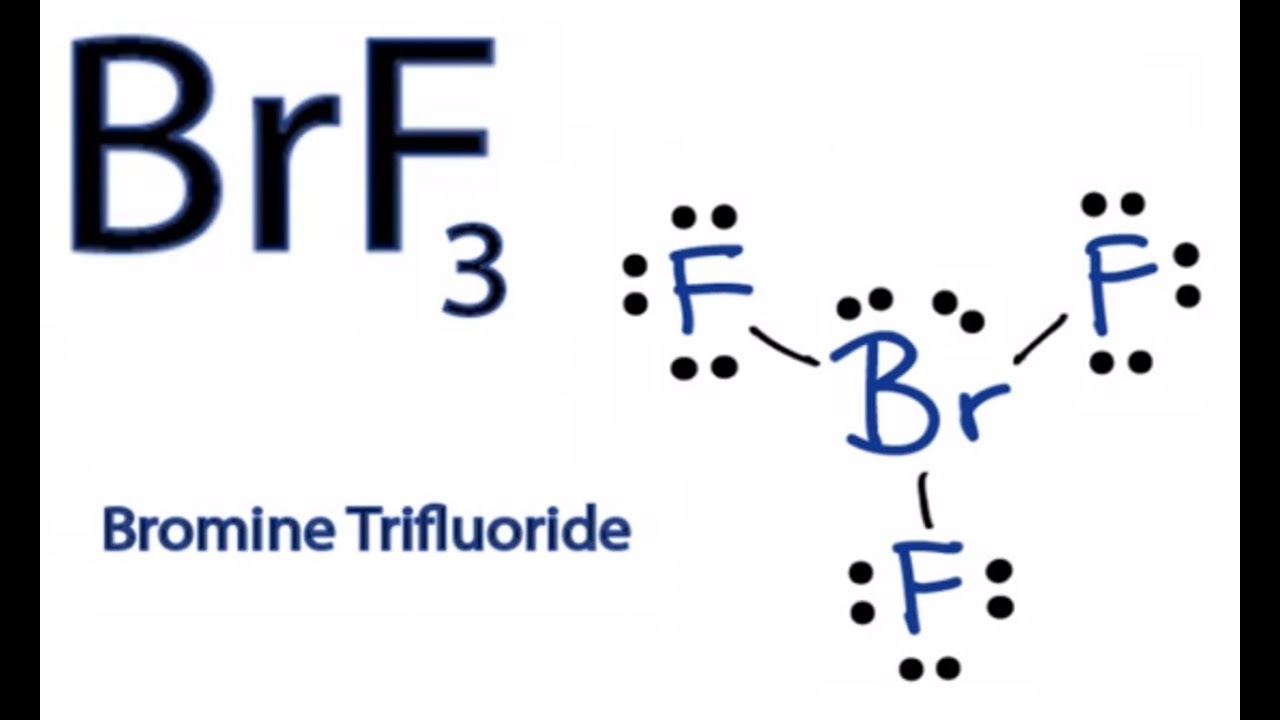
How To Draw The Lewis Dot Structure For Brf3 Boron Trifluoride Youtube
Hybridization Of Brf3 Hybridization Of Br In Bromine Trifluoride

Brf3 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
Brf3 Lewis Structure Draw The Bromine Trifluoride Dot Structure Geometry Of Molecules

Brf3 Lewis Structure Bromine Trifluoride Youtube

Brf3 Polarity Molecular Geometry Hybridization And Bond Angle Geometry Of Molecules

The Hybridization And Geometry Of Brf3 Molecules Are

Hybridization Of Brf3 Hybridization Of Xef2

Determine The Hybridization About Br In Brf3 A Sp B Sp 2 Clutch Prep
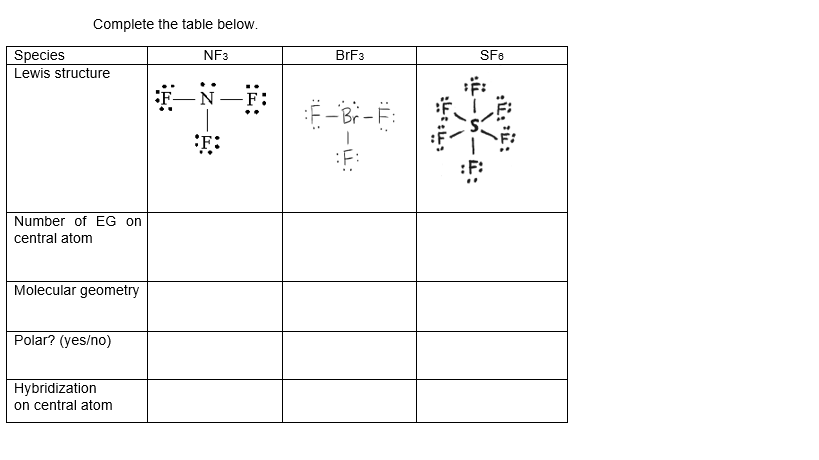
Complete The Table Below Species Lewis Structure Nf3 Chegg Com

Brf3 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

Hybridization Of Brf3 Bromine Trifluoride Youtube
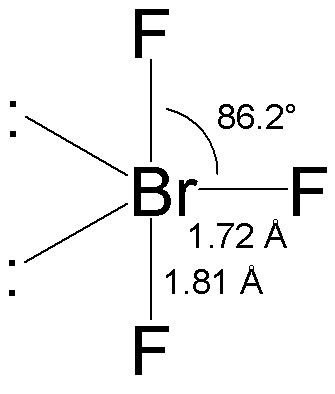
Brf3 Polarity Molecular Geometry Hybridization And Bond Angle Geometry Of Molecules

5 Draw The Most Appropriate Lewis Structure S For Brf3 Wh Clutch Prep