C2h2 Vsepr Structure
The structure of CO 2. In the lewis structure of C 2 H 4 there are only four C-H bonds one CC bond and no lone pairs on last shells.
Why Is An Ethene Molecule A Planar Molecule While Ethyne Is Linear Quora
Lets go ahead and draw this out.
C2h2 vsepr structure. σ framework π-bond Overall structure Question. We are interested in only the electron densities or domains around atom A. Thus its Lewis structure must account for 22 valence electrons.
How do you figure out the answer is linear. There are only single bond between carbon atom and hydrogen atom because hydrogen caannot keep more than two electrons in its last shell. When we talk about CH2Cl2 Carbon is less electronegative than Chlorine atoms.
What is the shape of CH3F. We have 2 carbons and we have 2 hydrogens. Acetylene was found in whole gas octane levels 87 89 and 92 at 00022 00032 and 00037 ppbC respectively 1.
The Answer is linear. For C 2 H 2 you have a total of 10 valence electrons to work with. Using Lewis Structures and the VSEPR model predict the molecular geometries of CO2 CH4 C2H2C2H4 and C2H6 and then use the geometries to describe the hybridization sp sp2 or sp3 expected for each carbon atom.
Ethyne is the chemical compound with the formula C 2 H 2. Best Lewis Structure bonding groups on central atom lone pairs of electrons on Name Kim Leibe VSEPR Shape. VSEPR theory explains the shape by minimizing the electronic repulsion.
To understand the Lewis structure lets first calculate the total number of valence electrons for Dichloromethane. For each multiple bond doubletriple bond subtract one electron from the final total. Since Fluorine is a very electronegative atom it makes CH3F a polar molecule.
This colorless gas lower hydrocarbons are generally gaseous in nature is widely used as a fuel and a chemical building block. View Notes - Oct_29 from CHEM 154 CHEM 154 at University of British Columbia. The CO2 molecule has 2 double bonds so minus 2 electrons from the final total.
We have this molecule this right here and we want its shape we want its molecular shape and also the geometry of electron pairs around its central atom. I know you draw the lewis structure but when I drew it I thought the answer would be tetrahedral because the carbon atom has 4 lone pairs around it. Total Domains Generic Formula Picture Bonded Atoms Lone Pairs Molecular Shape Electron Geometry.
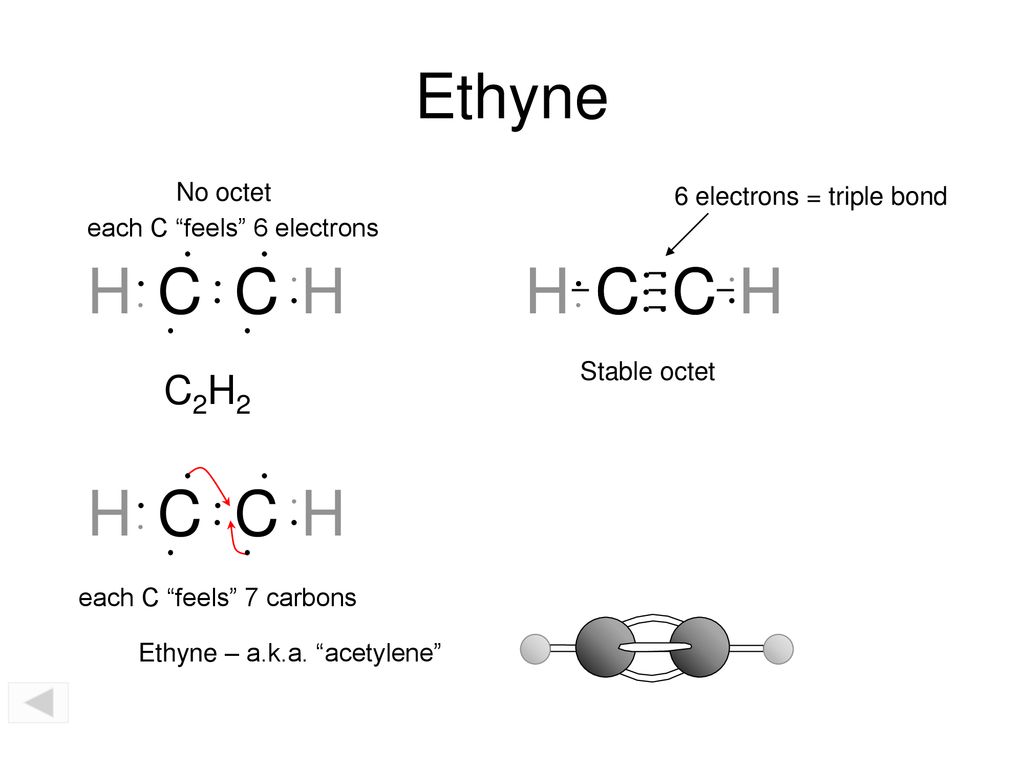
The solution is to share three pairs of valence electrons and form a triple bond between the Carbon atoms in C 2 H 2. It is a hydrocarbon and the simplest alkyne. Identify the σ framework and the π-bonds in acetylene C2H2 H-CC-H.
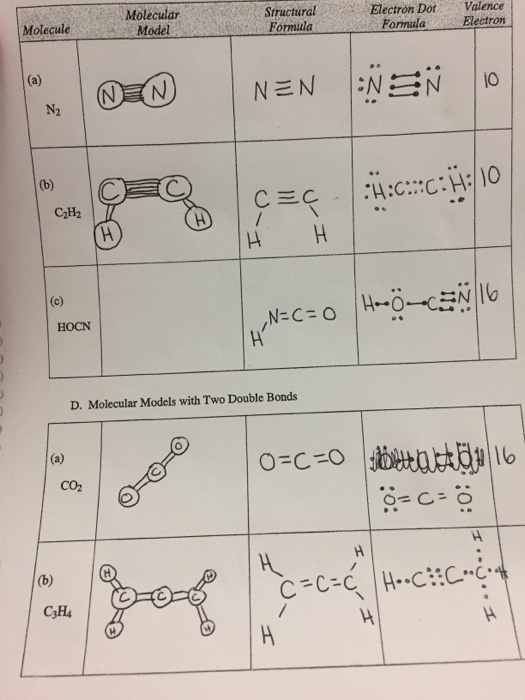
Molecule HOCN N2 C2H2 valence electrons 6N2 Molecular Models Data Sheet - Spring 2020 Multiple bonds. In C2H4 if we look into the lewis structure we will see that there are three bonded pairs of. Bonding in Acetylene C2H2 VSEPR predicts a linear structure Hybridization at C is sp bonds bonds Example Describe.
What is the shape of C2H2. We want molecular and electronic geometry of C2H2. The molecular shape of the C2Cl2 molecule is linear.
The molecular shape is predicted to be trigonal planar around each carbon atom. Here we need to deal with lone or unshared and bonded pairs of electrons. There are lone pairs on X or other atoms but we dont care.
Total number of Valence electrons 4 21 27. Starting with its Lewis structure the C2Cl2 molecule has a total of 22 valence electrons 4 from each of the two carbon atoms and 7 from each of the two chlorine atoms. CH3F Molecular Shape Yes CH3F is a tetrahedral with C creating four bonds as the central atom.
This is composed of a σ framework and a π-bond. Carbon has four valence electrons Hydrogen has one valence electrons and like all halogens Chlorine has seven valence electrons. VSEPR only recognizes groups around the central atom.
With two bonding pairs on the central atom and no lone pairs the molecular geometry of CO 2 is linear Figure 92. VSEPR Theory Molecular Shapes A the central atom X an atom bonded to A E a lone pair on A Note. Of course because carbons are in group 4A.
Use VSEPR theory to predict the molecular geometry around either carbon atom in acetylene C2H2. Acetylene systematic name. Therefore there cannot be more than one stable resonance structure for C 2 H 4.
Thus the lone pairs on the oxygen atoms do not influence the molecular geometry. The initial VSEPR shape for the CO2 molecule is Tetrahedral. This problem has been solved.
In drawing the Lewis structure for C 2 H 2 also called ethyne youll find that you dont have enough valence electrons available to satisfy the octet for each element if you use only single bonds. The C-F bond does affect something. Acetylene has been detected 12 29 and 50 of total hydrocarbon concentration in emissions from vehicle exhaust petroleum exhaust and petrochemical plants respectively 2.
Ethylene C2H4 has the Lewis Structure.
Molecule Molecular Model Structural Formula Electron Chegg Com
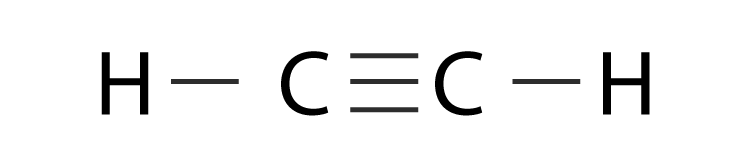
Hybridization Of C2h2 Hybridization Of C In Acetylene Ethyne
C2h2 Lewis Structure Molecular Geometry Hybridization Bond Angle
Is C2h2 Polar Or Non Polar Ethyne Or Acetylene Youtube

C2h2 Lewis Dot Structure Geometry Youtube

C2h4 Molecular Geometry Shape And Bond Angles Youtube
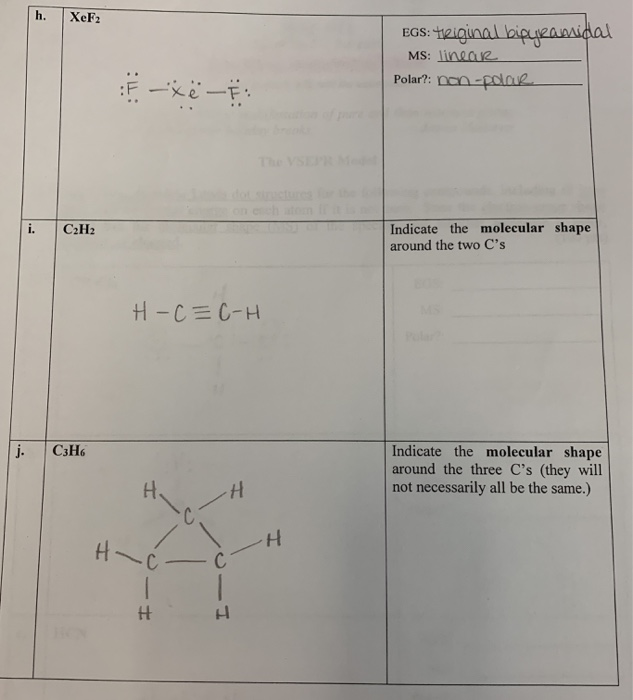
The Vsepr Model 1 Draw The Most Stable Lewis Dot Chegg Com
C2h2 Molecular Geometry Shape And Bond Angles See Description For Note دیدئو Dideo
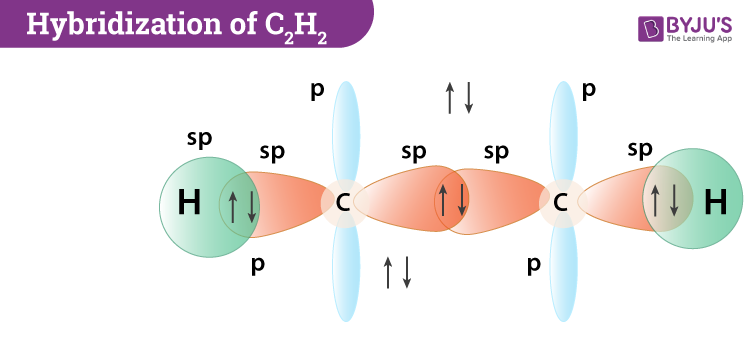
Hybridization Of C2h2 Hybridization Of C In Acetylene Ethyne

C2h2 Lewis Structure Molecular Geometry Hybridization Polarity And Mo Diagram Techiescientist
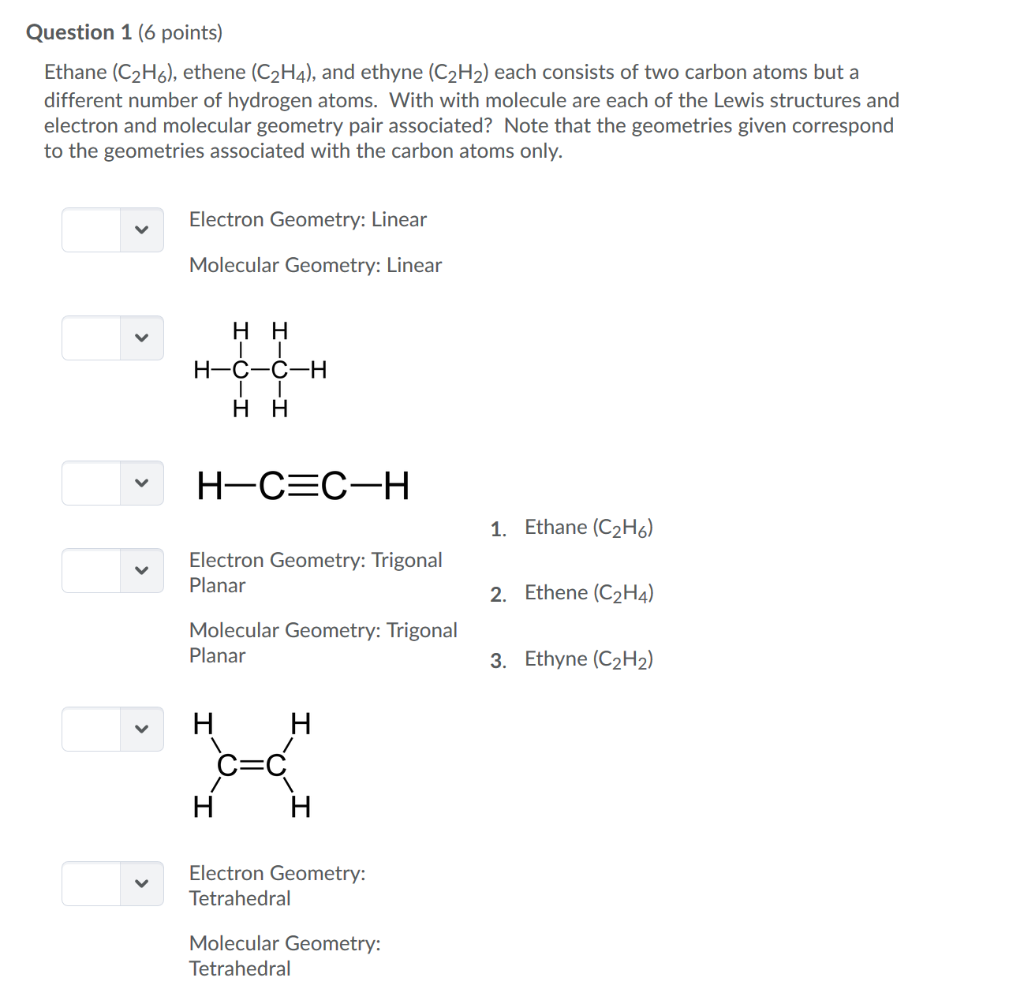
Question 1 6 Points Ethane C2h6 Ethene C2h4 Chegg Com

C2h2 Molecular Geometry Shape And Bond Angles See Description For Note Youtube
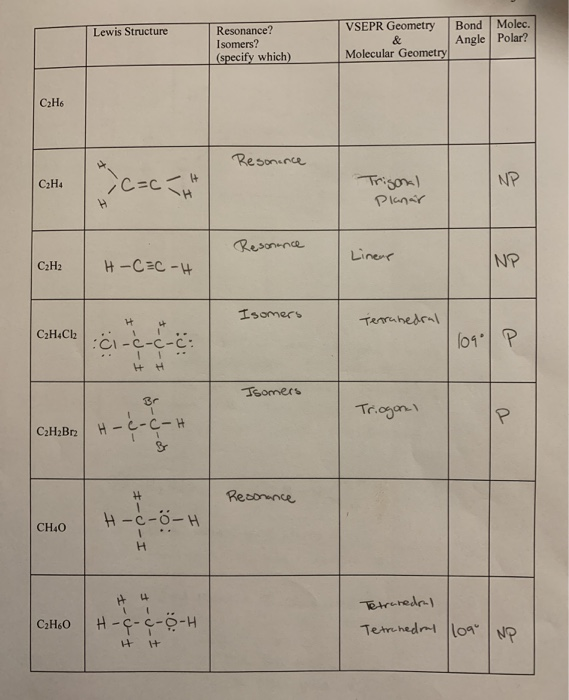
Lewis Structure Bond Molec Angle Polar Resonance Chegg Com

Molecular Geometry Of Acetylene Chemistry Stack Exchange

Molecular Models Vsepr Theory Polarity Datasheet Chegg Com
Https Www Toppr Com Ask En Ar Question How Many Molecules Are Planar Among The Following Moleculesi3 H2o

Ch 10 Vsepr Practice Problems 3 Flashcards Quizlet
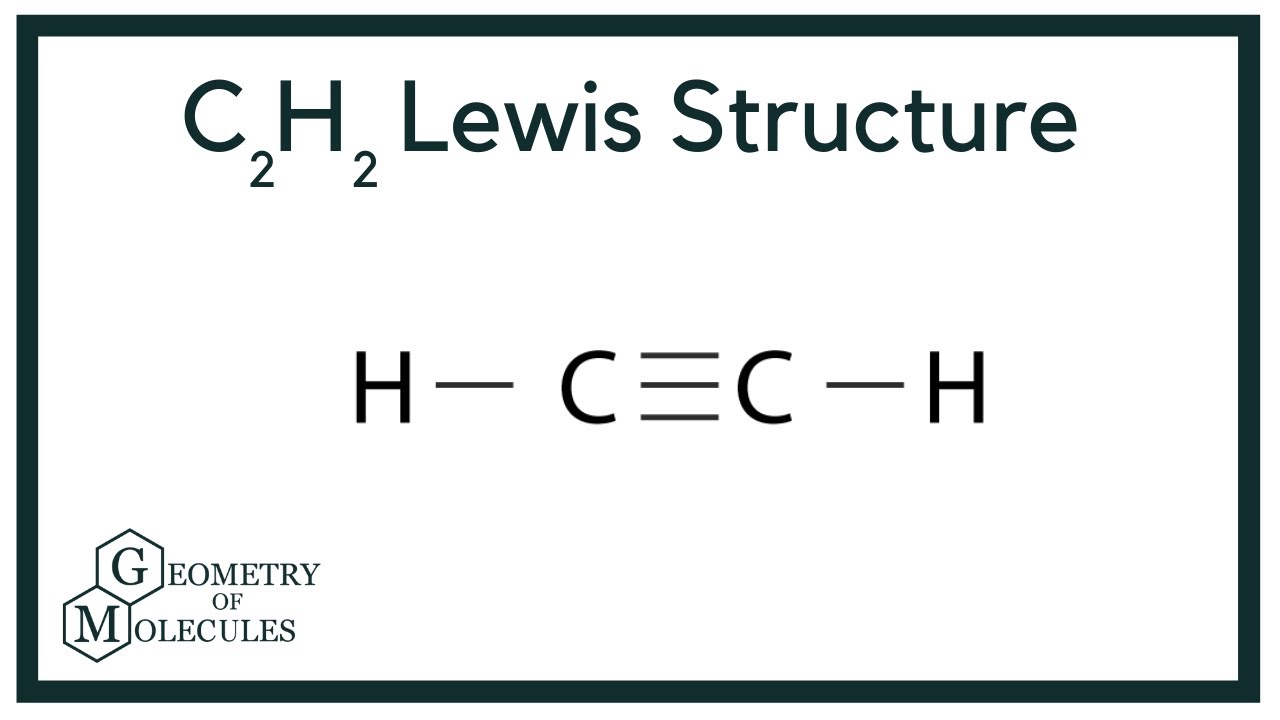
C2h2 Lewis Structure Molecular Geometry Hybridization Bond Angle
Molecular Models Activity Ppt Download