Cocl2 Lewis Structure Bond Angle
Claims of the formation of tri- and tetrahydrates have not been confirmed. H2S Total of Valence Electrons.
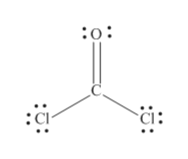
Answered What Is The Molecular Shape Of Coc12 Bartleby
Yes No Molecular Polarity.

Cocl2 lewis structure bond angle. COCl2 Hybridization Orbital hybridization is. The bond angle is 180o. Thus according to the VSEPR model the bonds are arranged linearly and the molecular shape of carbon dioxide is linear.
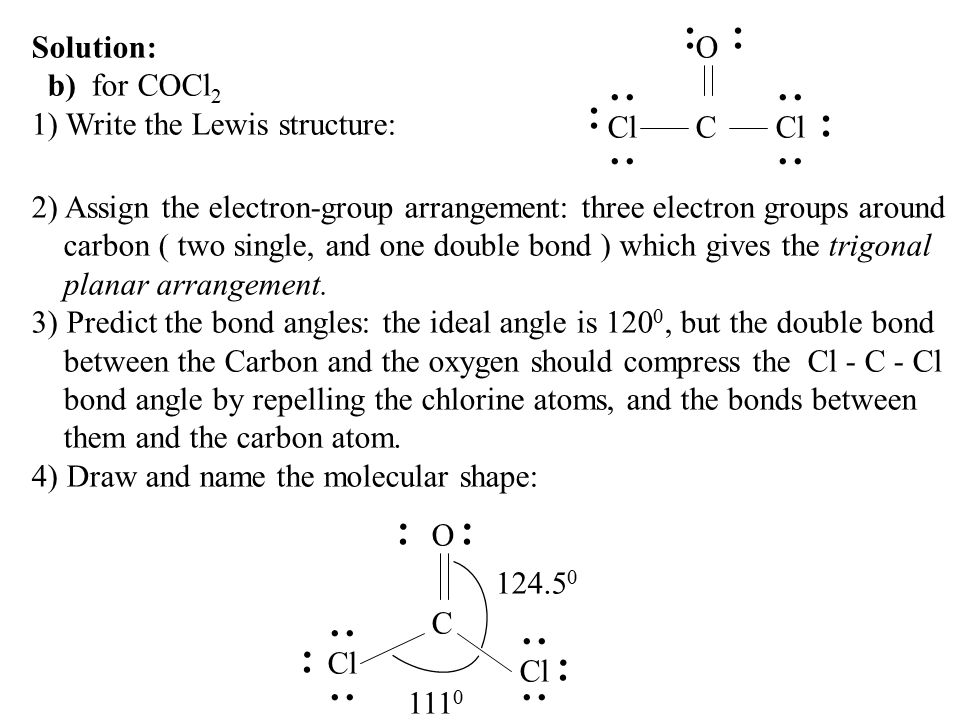
The bond angle of C-Cl bonds is around 1118 degrees less than 120 degrees due to CO electron density that reduces the bond angle. Chemistry questions and answers. Thus the molecular shape of COCl2 is trigonal planar.
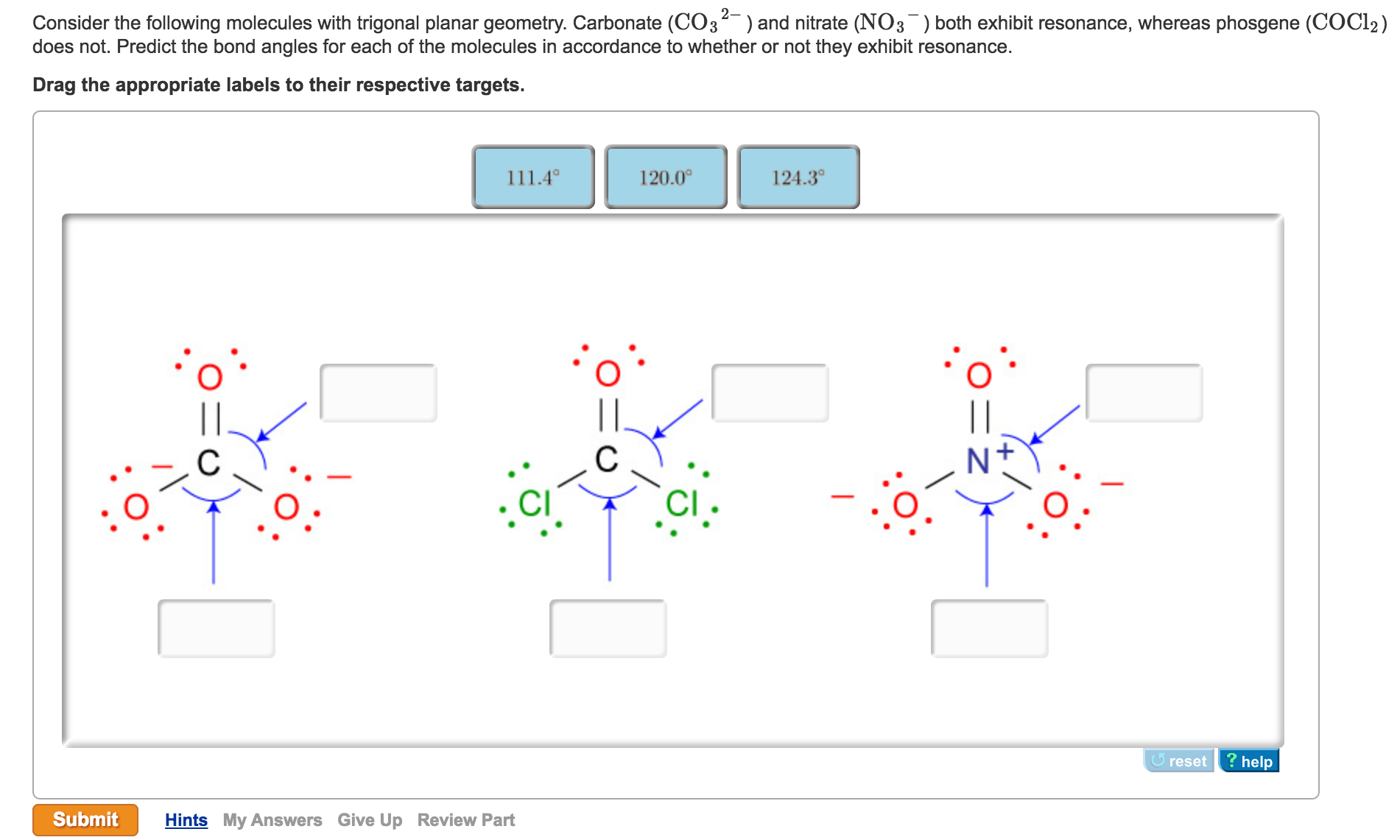
Since the double bond can be placed in more than one place without rearranging the atoms COCl2 exhibits resonance. The bond angle is 180o. Explain briefly why you believe estimate bond angle is Smaller or Larger than VSEPR Idealized bond angle.
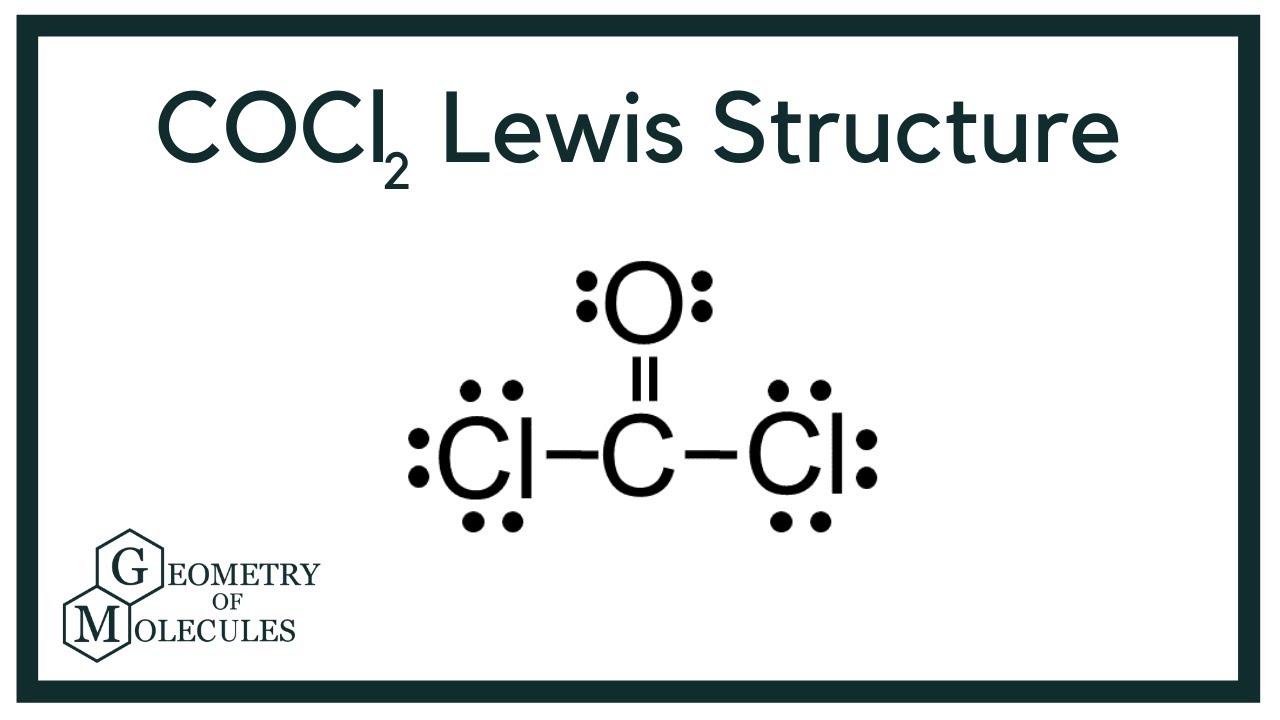
Is the COCl 2 molecule polar or nonpolar. Draw the Lewis structure for COCl2 including lone pairs. CoCl2 has a Linear molecular structure with bond angles of 180.
This molecule has an AX2general formula with 2 bonding pairs no lone pairs. Drawing and Evaluating the Structure of COCl2 025 pt per each blank for 3a and 36 1 pe for 3e 125 1 1 325 pts a. 012 997 2882 navraedoringenrosiecoza.
Does COCl2 have resonance structures. What is the ClCCl bond angle. An explanation of the molecular geometry for the COCl2 ion Phosgene including a description of the COCl2 bond angles.
The Lewis structure of the CoCl2 molecule shows that there is a double bond between the O and the central C atom. You have two double bonds or two electron groups about the carbon atom. Explain briefly your choice.
A double bond represents a slightly larger electron domain which disrupts the ideal 120 CI-C-Cl bond angle making it smaller. A molecule has resonance if more than one lewis structure can be drawn for that molecule. 3-D Model Sketch Bond Angles Lewis Structure show all resonance structures if applicable Molecular Shape.
CoCl2 Lewis Structure Molecular Structure Hybridization Bond Angle and Shape 16 Jul C2H4 Lewis Structure Molecular Structure Hybridization Bond Angle and Shape. COCl2 is a polar molecule because the dipole between the carbon and the chlorine atoms is not equal to the dipole between the carbon and oxygen atoms. Since there is only one possible lewis structure C2H2 does not have resonance.
A quick explanation of the molecular geometry of SOCl2 including a description of the SOCl2 bond anglesLooking at the SOCl2 Lewis structure we can see that. It can be studied with the help of Valence Bond Theory VBT which confirms that sp2 hybridization is only possible when the molecular geometry is trigonal planar. The three groups of electron pairs are arranged in a trigonal.
Any polar bonds in the molecule. Does C2H2 have resonance. The Lewis structure of the COCl2 molecule shows that there is a double bond between the O and the central C atom.
As we can find out the 3D geometry of COCl2 is trigonal planar. The COCl2 molecule has 3 areas of electron repulsion around the central C atom so the shape is trigonal planar. Lewis Structures and Molecular Shapes 1.
As the molecular geometry of carbonyl fluoride COF2 is trigonal planar with the bond angle of 120 with no distortion from the ideal state the hybridization of the central atom is sp2. Bond angle is 120o. A double bond represents a slightly larger electron domain which disrupts the ideal 120 Cl-C-Cl bond angle.
What is the Cl - C - Cl bond angle. The C - Cl bond in COCl2 is polar or nonpolar. In order to explain the observed geometry bond angles that molecules exhibit we need to make up hybridize orbitals that point to where the bonded atoms and lone pairs are located.
Both CCl bonds are polar due to the difference in electronegativity of C and Cl. Bond angle in COCl 2 Bond angle in COCl 2 molecule is 120ºThe representation is shown below. The electron geometry for the Phosgen.
Draw the Lewis structure for COCl 2 including lone pairsWhat is the molecular shape of COCl 2Is the CCl bond in COCl 2 polar or nonpolar. The molecule COCl2 is polar or nonpolar. What is the molecular shape of COCl2.
Arrangement - AX3 with 3 bonding pairs no lone pairs on the central atom.

Chapter 10 The Shapes Of Molecules Ppt Download
Chapter 10 The Shapes Of Molecules Ppt Video Online Download
Consider The Following Molecules With Trigonal Planar Chegg Com
Vsepr Shape Of Cocl2 Biochemhelp

Cocl2 Phosgene Molecular Geometry Bond Angles And Electron Geometry Youtube
Cocl2 Lewis Structure Phosgene Youtube
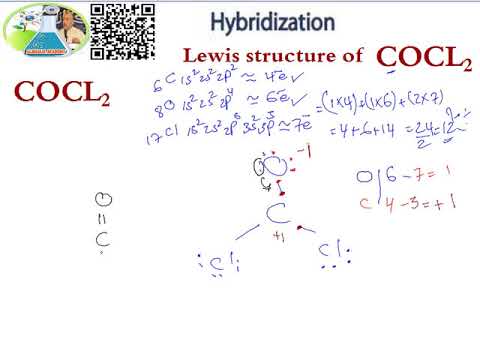
Lewis Structure Hybridization Cocl2 Youtube
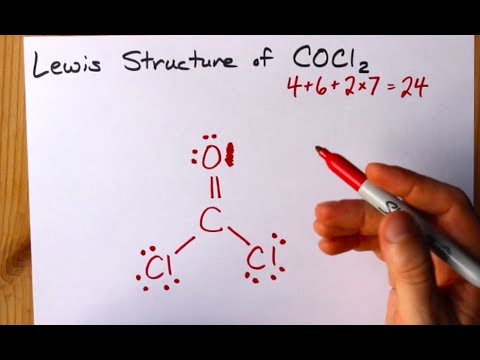
How To Draw The Lewis Structure Of Cocl2 Dichloromethanal Phosgene Youtube
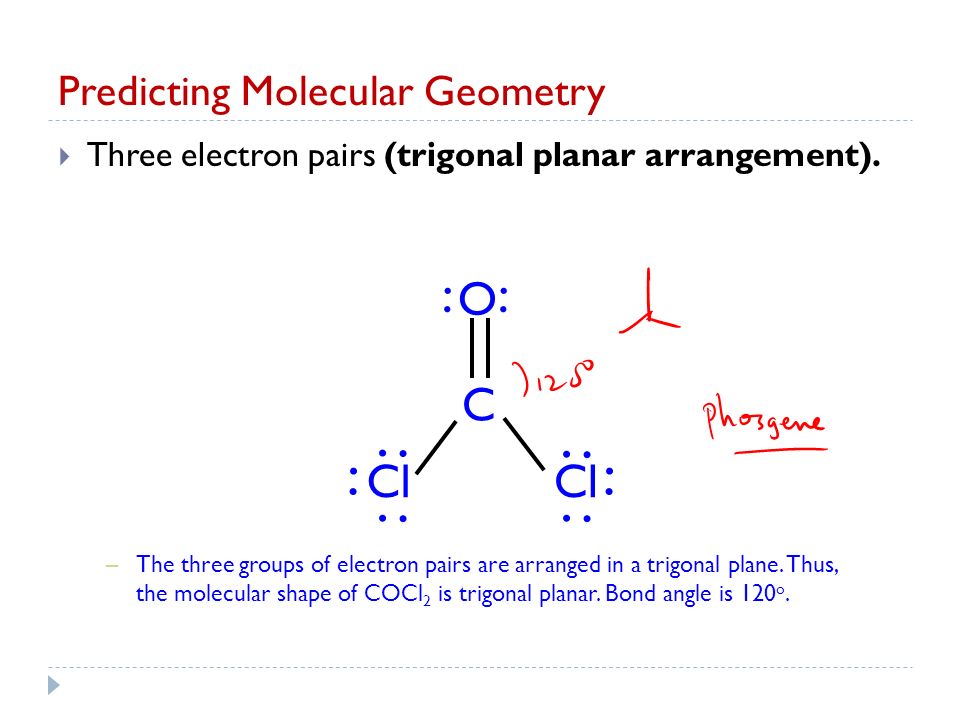
Carvone Bucky Ball Molecular Geometry Chapter 8 Part Ppt Video Online Download

Chemical Bonding Ii Molecular Geometry And Hybridization Of Atomic Orbitals Chapter Ppt Download

Cocl2 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Cocl2 Lewis Structure How To Draw The Lewis Structure For Cocl2 Youtube

Cocl2 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Cocl2 Lewis Structure How To Draw The Lewis Structure For Cocl2 Youtube


