Electron Dot Structure For Nh3
Which molecular geometry is least likely to occur with sp3 hybridization. As a result bonding pairs of electrons push away nonbonding pairs of electrons to form a pyramidal shape.
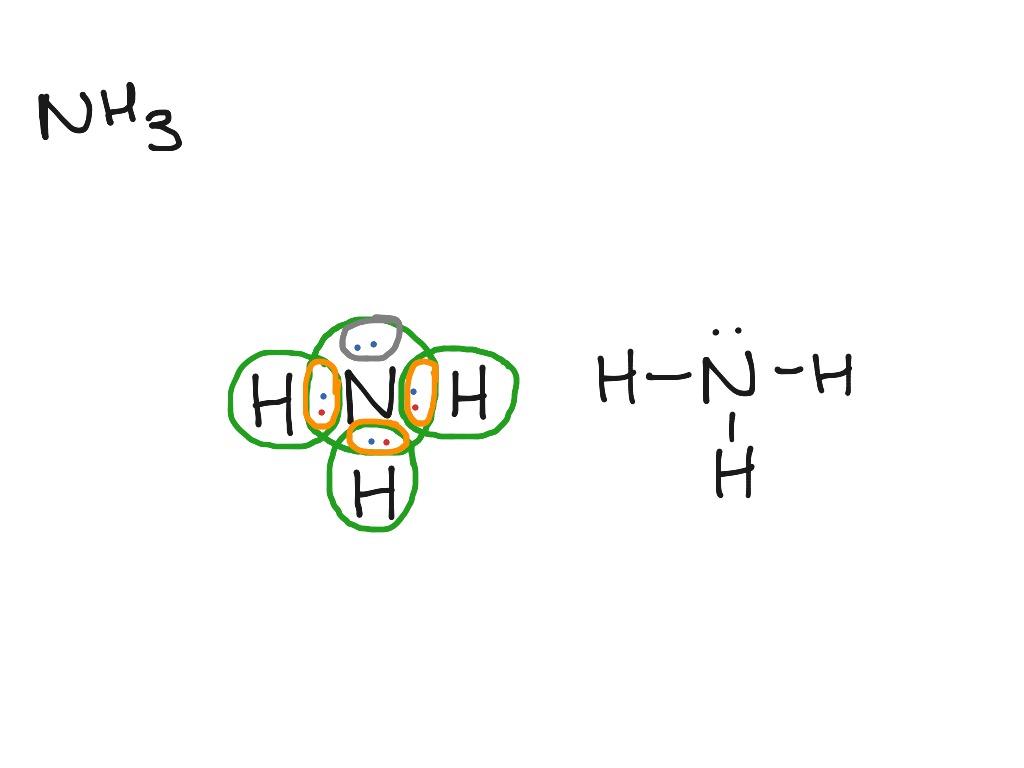
Lewis Diagram Nh3 Science Chemistry Molecules Showme
Photograph SharkRay metabolic waste.

Electron dot structure for nh3. There are three single bonds and one lone pair of electrons in NH3 molecule. Photograph NH3 Lewis and 3-D Structure- Dr. The lewis structure for nh3 is one of the most common lewis structures to show up on.
It then is single bonded to three hydrogen atoms and has one lone pair. In the nh 3 lewis structure and all structures hydrogen goes on the outside. Electron Dot Structure of NH3 by Jeff Bradbury - February 17 - Lewis Electron Dot Structure for ammonia molecule NH3.
Lewis structure of NH3. Nitrogen goes in the centre. Lewis Structure of NH3.
Draw electron dot structure of co2 and NH3 - Brainlyin. Electron dot diagram for nh3. Electron dot structure of nh3 by jeff bradbury february 17 2012 lewis electron dot structure for ammonia molecule nh3.
Calculate the total valence electrons in the molecule. Photograph Lewis Structures Review Set 3. Electron dot structure of nh3 by jeff bradbury february 17 2012 lewis electron dot structure for ammonia molecule nh3.
The Lewis structure of ammonia NH3 would be three hydrogen atoms bonded to a nitrogen atom in the middle with a lone pair of electrons on top of the atom. The Lewis structure of NH3 is made in such a manner that the scarcity of one valence electron in each hydrogen atom total three hydrogen atoms as well as three valence electrons in the nitrogen atom is fulfilled and balanced. The unbonded electrons are called lone pairs of electrons.
Nh 3 ammonia is a commonly tested lewis structure. Nitrogen has 5 electrons in the valence shell so it needs to combine with 3 hydrogen atoms to fulfill the octet rule. These arrange in a tetrahedral geometry.
The shape is distorted because of the lone pairs of electrons. After determining how many valence electrons there are in NH3 place them around the central atom to complete the octet. For the NH3 Lewis structure calculate the total number of valence electrons for the NH3 molecule NH3 has 8 valence electrons.
However in reality hydrogen atom is rather prone to. By crator-avatar Jeff Bradbury 2. I2 draw electron dot structure for magnesium draw electron dot structure for nh3 draw electron dot structure of carbon tetrachloride.
Five plus 3 a total of 8 valence electrons. Alternatively a dot method can be used to draw the NH 3 Lewis structure. Remember that uncharged nitrogen has 3 bonds and one lone pair and hydrogen has one bond and no lone pairs.
Photograph Electron Dot Structures. In the formal way we find how many electrons we have step 1 how many each atom needs step 2 how many of those are bonding step 3 4 and how many are lone pairs step 5. This pair exerts repulsive forces on the bonding pairs of electrons.
Technically both the structures ceH2N-OH and ceH3Nbond-O may exist. These arrange in a tetrahedral geometry. The electron-dot structure Lewis structure for which of the following molecules would have two unshared pairs of electrons on the central atom.
Photograph The Big Picture combining spirituality science and. The Lewis structure of ammonia NH3 would be three hydrogen atoms bonded to a nitrogen atom in the middle with a lone pair of electrons. Lewis Electron Dot Structure Nh3 113 What are Lewis structures CH4 Lewis Structure How to Draw the Dot Structure for MakeTheBrainHappy.
Lewis Electron Dot Structure For Nh4 Formal Charge Problems 1 NH4 YouTube Coordinate Covalent Bond Chemical Bond Lewis Structure phosphine Wikidata Electron Dot Structures. The electron dot structure of NH3 has a nitrogen atom in the center. Draw electron dot structures for each molecule draw electron dot structures for atoms of the following elements draw electron dot structure for the following molecules draw electron dot structure for h2s draw electron dot structures for the following substances.
The Lewis Dot Structure for NH3 ShowMe Lewis electron dot diagram for CH. It has a molecular geometry of trigonal pyramidal which also looks like a distorted tetrahedral structure. Photograph Exam 3.
NH3 has trigonal pyramidal geometry because of the presence of lone pair of electrons with the central nitrogen atom. Furthermore what is the shape of nh3. This info can then be used to determine the.
This is the reason why ammonia acts as a Lewis base as it can donate those electrons. A step-by-step explanation of how to draw the NH3 Lewis Dot Structure AmmoniaFor the NH3 structure use the periodic table to find the total number of vale.

Nh3 Lewis Structure Molecular Geometry What S Insight
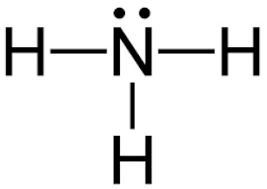
By Looking At The Lewis Dot Structure Of Ammonia Nh3 Class 11 Chemistry Cbse
Draw The Electron Dot Structure Of O2 Nh3 And Ccl4 Science Carbon And Its Compounds 10050281 Meritnation Com

Dot Structure Of Nh3 Brainly In

What Is The Electron Dot Structure Of Ammonium Chloride Brainly In
Nh3 Lewis Structure Ammonia Youtube
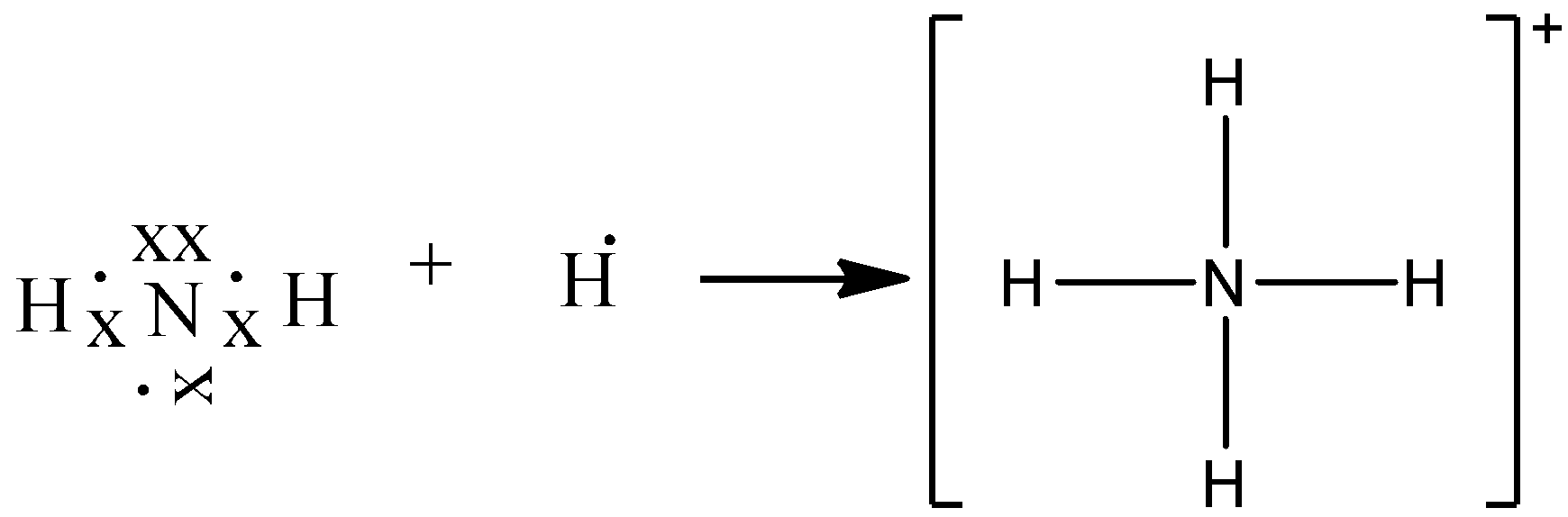
Draw An Electron Dot Diagram To Show The Formation Class 11 Chemistry Cbse
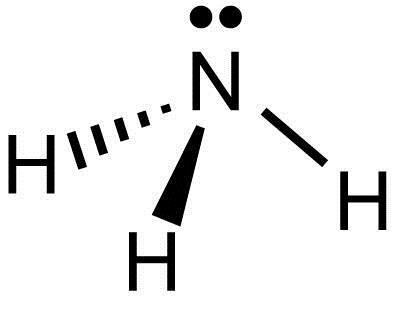
What Is The Lewis Structure Of Nh3 Socratic

How To Draw Lewis Structures Youtube

File Nh3 Bcl3 Adduct Bond Lengthening 2d Png Wikipedia

In The Space Provided Below Draw Electron Dot Diagrams For The Following Molecules Hydrogen H2 Brainly Com

Lewis Structure Nh3 Plus Dipoles Shape Angles And Formal Charge Youtube
Lewis Electron Dot Structures Ck 12 Foundation

Lewis Structures 2 Water And Ammonia Youtube

Nh3 Lewis Structure Geometry And Hybridization Techiescientist
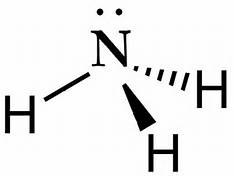
How Is Ammonia Represented By An Electron Dot Diagram Socratic

Nh3 Lewis Structure Ammonia Youtube
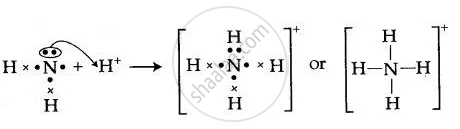
By Drawing An Electron Dot Diagram Show The Lone Pair Effect Leading To The Formation Of Ammonium Ion From Ammonia Gas And Hydrogen Ion Chemistry Shaalaa Com

