H2so4 Lewis Structure Resonance
70 more lewis dot structures. Stream Producer Q t 4.
Lewis Structure Of Sulfuric Acid H2so4 Steps Of Drawing
A step-by-step explanation of how to draw the HSO4- Lewis Dot Structure Bisulfate ion or Hydrogen sulfate ionWhen we have an H or H2 in front of a polya.
H2so4 lewis structure resonance. Hydrogen is IA group element. The sulfuic acid is 950 H2SO4 by mass and has a density of 184gL. Both Sulfur and oxygen atoms are located at VIA group in the periodic table.
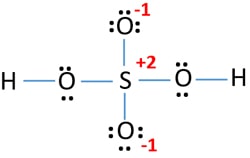
This the lewis dot structure of h2so4. Therefore it has only one electron in its last shell. When 2 forms are non-equivalent they contribute in proportion to their acceptability.
We start with a valid Lewis structure and then follow these general rules- Resonance. Draw The Lewis Structure For H2so4 lewis structure draw lewis structures acid sulfuric electrons valence structure resonance draw octet rule many bonded shapes each brainly lewis structure drawing begingroup stack lewis acid sulfuric draw structures bonded. There are equivalent six resonance structures SO4 2- the Sulfate ion.
One hydrogen atom is joint to phosphorous atom and remaining hydrogen atoms are joint to. In the lewis structure for h 2 so 4 there are a total of 32 valence electrons. Two of the oxygens are single-bonded and two are double-bonded.
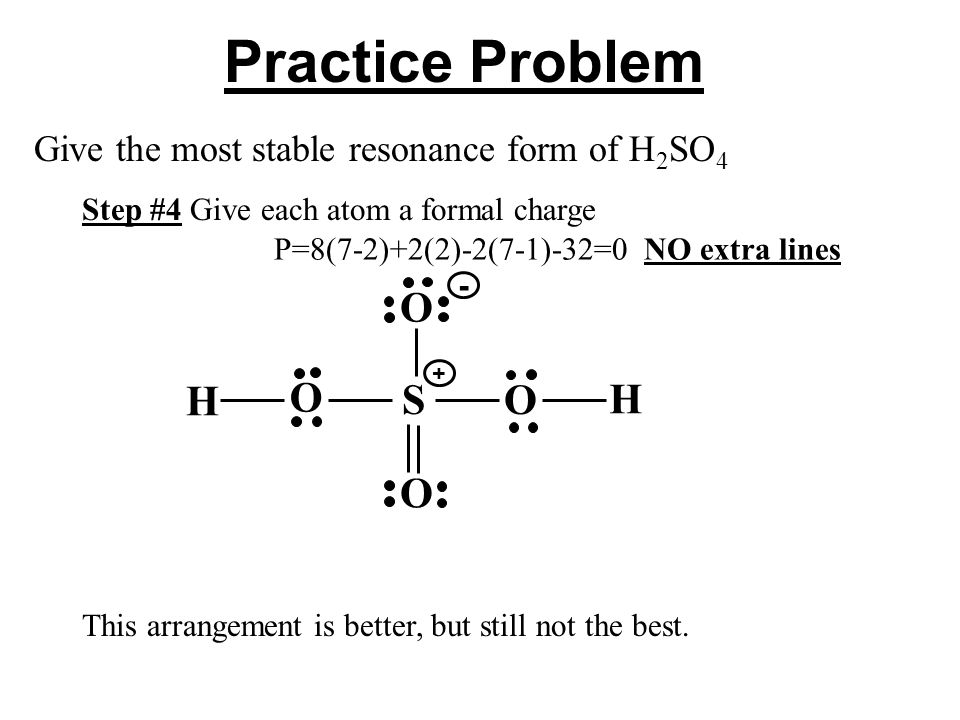
Total number of electrons of the valance shells of H 2 SO 4. Because the formal charges are close to zero with this structure that makes this the more likely Lewis structure for IO3-. Each cl atom interacts with eight valence electrons total.
Sodium Carbonate Na2CO3is used to neutralize the Chemistry You have the following acids and their conjugate bases available. I quickly take you through how to draw the Lewis Structure of H2SO4 Sulfuric Acid. So oxygen and sulfur atoms have six electrons in their valence shells.
There are three single bonds in the molecule. We start with a valid Lewis. Procedure for developing resonance forms applied to H 2 SO 4.
HNO3 NO2 H2SO4 HNO3 NO2 H2SO4 asked Aug 22 2018 in Chemistry by Sagarmatha. Sulfur make six bonds in this Lewis Structure. Hno3 no2 h2so4 asked aug 22 2018 in chemistry by sagarmatha 544k points.
Write lewis structure of the following compounds and show formal charge on each atom. For H2SO4 molecule sulfur has the highest valence than oxygen and hydrogen. Ka 42 10-7 Hydrofluoric acid.
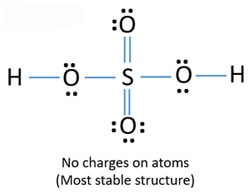
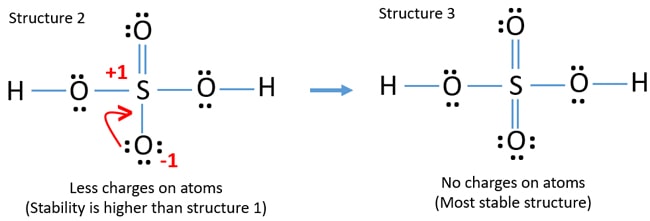
The actual structure is more stable than either of the hypothetical contributing forms. H2so4 lewis structure h2so4 lewis structure angle h2so4 lewis structure charge h2so4 lewis structure resonance h2so4 lewis structure tetrahedral h2so4. 2 ikatan kovalen tunggal c.
The lowest energy geometry is the traditional H X 2 S O X 4 Lewis structure estimated at Δ H f 0 -17788 kcalmol. I also go over hybridization shape and bond angles. These are some keyword suggestions for the term h2so4 lewis structure.
These structures are known as resonance structures. These structures exhibit similar properties. When 2 resonance forms are equivalent they contribute equally to the true structure.
We could go on expanding the s octet but this would start to move the formal charges away from zero. H2so4 lewis structure molecular geometry. Not all resonance structures are equal there are some that are better than others.
It will hold more than 8 electrons. Write Lewis structure of the following compounds and show formal charge on each atom. While PM7 isnt a highly accurate method the difference in stability isnt close.
Now we can find the total valence. The rearrangement of electrons lone pair and double bonds in the Lewis structure of compound gives different structures. Ka 18 10-5 Carbonic Acid.
SMask None Therefore only. Hill or High Water 5k Run 1 Mile Fun Walk Hill or High Water 5k Run 1 Mile Fun Walk. Drawing the correct lewis structure is important to draw resonance structures correctly.
The key to understanding this structure of Lewis is that you have these H ahead and then you have this polyatomic ion. There are no charges at any atom. Ka 72 10-4 Which of these.
The reason is FORMAL CHARGE and the fact tha. Je comprends pas un truc sur la formule de lewis de h2so4. Lewis structure of phosphoric acid contains -1 charge on one oxygen atom and 1 charge on phosphorous atom.
There are two lewis structures for.

Which Is Stronger Acid H So Or H So And Why Quora

H2so4 Lewis Structure Sulfuric Acid Youtube

Lewis Structure Of Sulfuric Acid H2so4 Steps Of Drawing

Lewis Structures H2so4 Youtube
Lewis Structure Of Sulfuric Acid H2so4 Steps Of Drawing

Formal Charges And Resonance Chemistry For Majors Atoms First
Chapter 8 Chemical Bonding Ppt Video Online Download
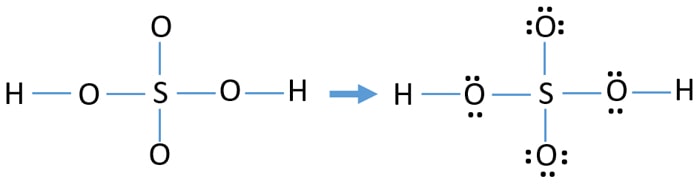
Lewis Structure Of Sulfuric Acid H2so4 Steps Of Drawing

How Many Resonance Structures Would H2so4 Have Study Com

Lewis Structures H2so4 Youtube
4 8 Chemical Bonding And Molecular Geometry Exercises Chemistry Libretexts
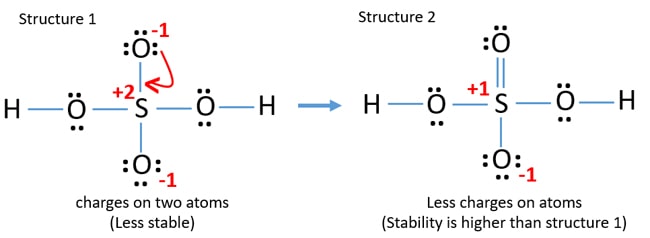
Lewis Structure Of Sulfuric Acid H2so4 Steps Of Drawing

H2so4 Lewis Structure How To Draw The Dot Structure For Sulfuric Acid
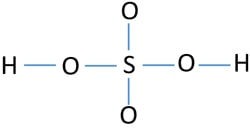
Lewis Structure Of Sulfuric Acid H2so4 Steps Of Drawing
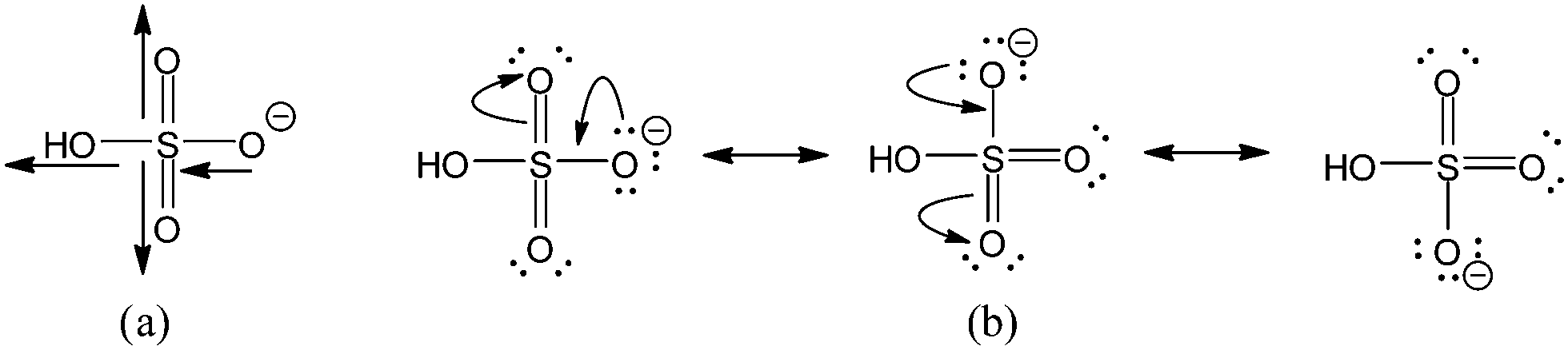
Why Is Sulfuric Acid A Much Stronger Acid Than Ethanol Determination Of The Contributions By Inductive Field Effects And Electron Delocalization Effe Physical Chemistry Chemical Physics Rsc Publishing Doi 10 1039 C4cp04110k

Lewis Dot Structure Of H2so4 Brainly In

Draw The Lewis Dot Structures Of A H2so4 B Co3 2 C O3
