How Many Valence Electrons Does C2h2 Have
Sodium will loose 1 electron. From the Lewis structure one can see that there is a triple bond between the two Cs.

36 The Chemical Formula Of Acetylene Is C2h2 How Chegg Com
Drawing the Lewis Structure for C2H2 Ethyne or Acetylene For C2H2 you have a total of 10 valence electrons to work with.
How many valence electrons does c2h2 have. And this Carbon has 2 4 6 8. In drawing the Lewis structure for C2H2 also called ethyne youll find that you dont have enough valence electrons available to satisfy the octet for each element if you use only single bonds. Best Lewis Structure bonding Groups On Central Atom lone Pairs Of Electrons On VSEPR Shape.
HOCN CzH NH3OH NH H2O Molecule valence Electrons 6N2 Multiple Bonds. Once you get the total number of valence electrons you can make a Lewis dot structure of HCN. The carbon atoms are bonded to each other and each hydrogen atom is bonded to a carbon atom.
The solution is to share three pairs of valence electrons and. Question How many valence electrons does magnesium have. C gets 6 electrons and O gets 8 electrons in their outer shell respectively.
In drawing the Lewis structure for C2H2 also called ethyne youll find that you dont have enough valence electrons available to satisfy the octet for each element if you use only single bonds. And if we count them all up we have 2 4 6 8 10 valence electrons which is what we had to start with and weve written the Lewis structure for C2H2. In the case of carbon we have four valence electrons each.
A molecule has resonance if more than one lewis structure. Selection of center atom and sketch of C 2 H 2 molecule To be the center atom ability of having greater valance and being a electropositive element are important facts. In drawing the Lewis structure for C2H2 also called ethyne youll find that you dont have enough valence electrons available to satisfy the octet for each element if you use only single bonds.
The solution is to share three pairs of valence electrons and form a triple bond between the Carbon atoms in C2H2. C has four valence electrons two in 2s and two in 2p and H has one one in 1s. Answer Magnesium has just two valence electrons and 12 electrons.
So in order to achieve the 8 electrons in the outer shell Carbon takes a lone pair from Oxygen thus making a non-covalent bond. We can clean it up a little bit to make it easier to see here. This problem has been solved.
There is a total of ten valence electrons. Sothe total number of C2H2 valence electrons is ten 8 2 10 These 10 valence electrons have two tasks at the the same timeThey connect all the atoms and satisfy the duet rule of hygrogens and octet rule of two carbon atoms. Hydrogen is the first element in the periodic table therefore it has only one valence electron.
The total number of valence electrons in one molecule of C2H4 2414 12. This structure helps in understanding the arrangement of valence electrons around the atoms in the molecule. Show transcribed image text.
A step-by-step explanation of how to draw the C2H2 Lewis Dot Structure Ethyne or AcetyleneFor the C2H2 structure use the periodic table to find the total. There are 2 types of elements in the periodic table. For C 2 H 2 total pairs of electrons are 5 102 in their valence shells.
Since there is only one possible lewis structure C2H2 does not have resonance. Ethylene C2H4 involves a double bond between the carbons so each carbon devotes two electrons to its neighboring carbon and two electrons to hydrogens. And you notice all those valence electrons being shared in the center.
Metals are the elements which have a tendency to loose electrons and thus they form cations. How many valence electrons are in lone pairs in the Lewis dot structure of C2H2. Previous question Next question.
Ethyne C2H2 has a triple bond between the two carbon atoms. Magnesium is classified as a metal. For each C one can explain the bonds through sp hybridization a triple bond and one single bond.
How Many Valence Electrons Does C02 HaveWhat is the number of valence electrons in co2How many valence electrons are in a carbon dioxide co2 molecule. However from our experience we know that there is a very low possibility that hydrogen being a center atom in a molecule. The linear acetylene molecule C2H2 is formed by carbon atoms which each share three of their four valence electrons with each other a structure called a triple bond.
C 2 H 2. It has an octet. Is c2h2 a triple bond.

C2h2 Lewis Structure Molecular Geometry Hybridization Bond Angle
C2h2 Lewis Structure Tutorial How To Draw The Lewis Structure For Ethyne Or Acetylene دیدئو Dideo

Lewis Structure For C2h2 Ethyne
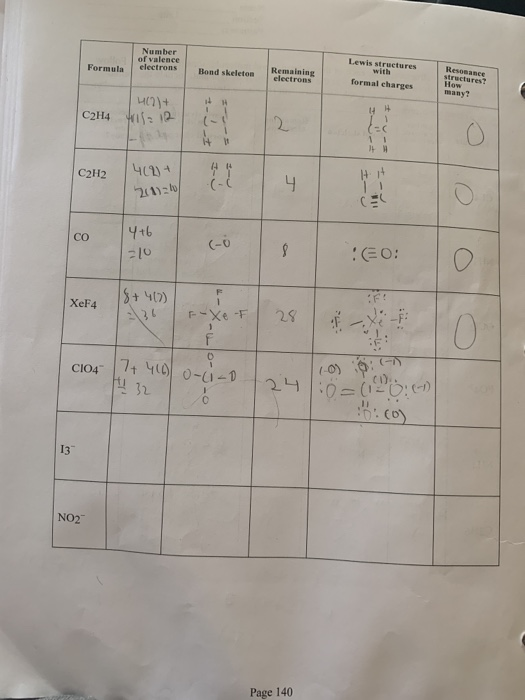
Lewis Structures Number Of Valence Electrons Ressance Chegg Com
Ch4 C2h4 C2h2 Total No Of Valence Electrons Lewis Chegg Com
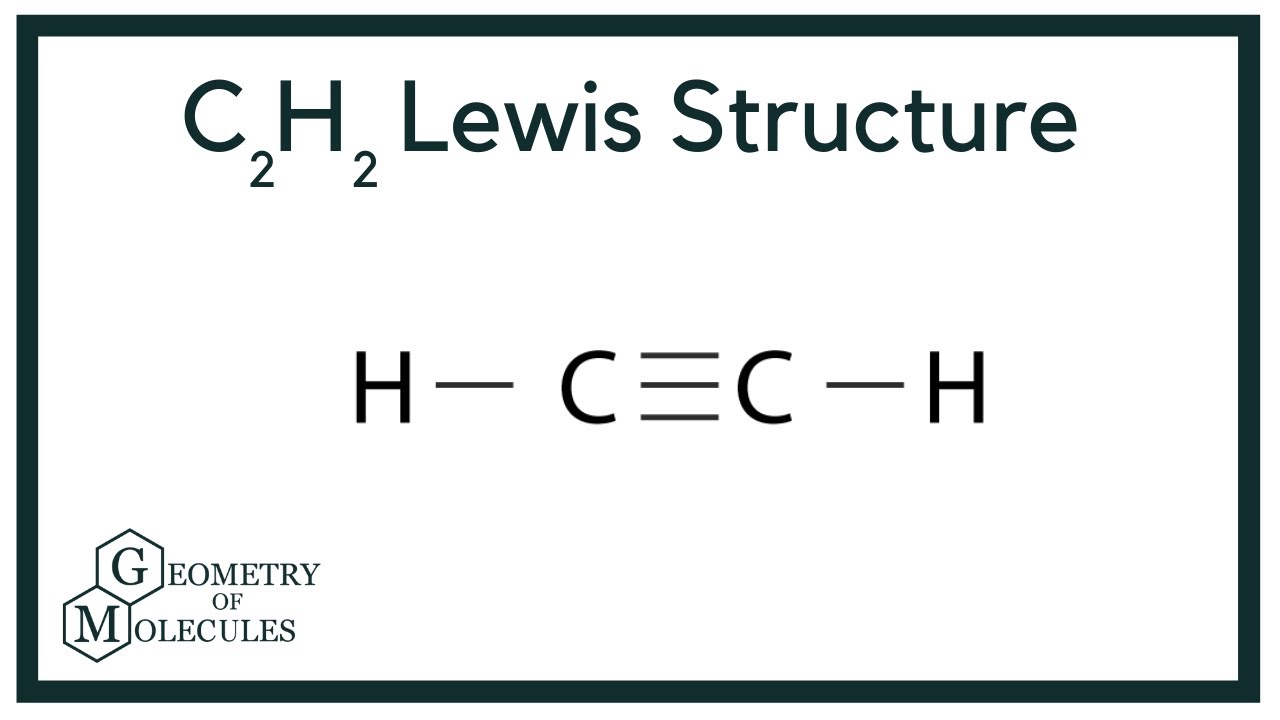
C2h2 Lewis Structure Molecular Geometry Hybridization Bond Angle
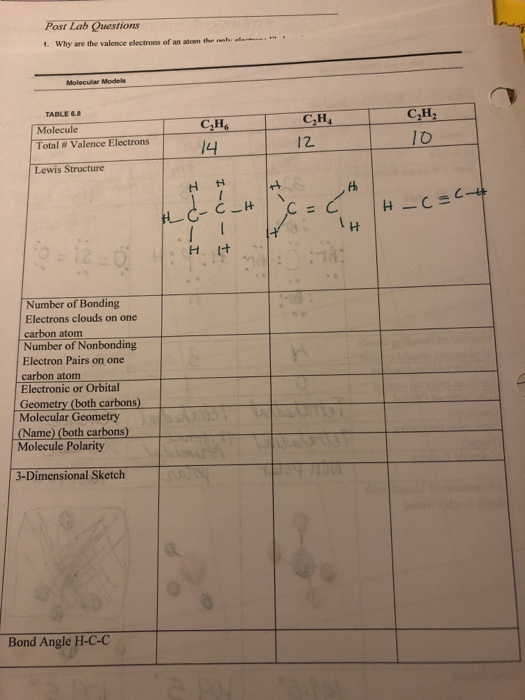
Post Lab Questions 1 Why Are The Valence Electrons Chegg Com
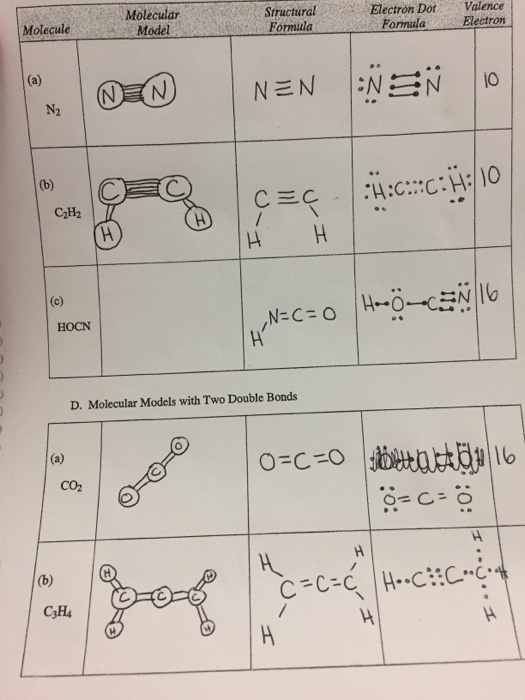
Molecule Molecular Model Structural Formula Electron Chegg Com

What Is The Lewis Structure Of C2h2 Study Com

Hcch Lewis Structure How To Draw The Lewis Structure For The Hcch Youtube

C2h2 Lewis Structure Molecular Geometry Hybridization Polarity And Mo Diagram Techiescientist
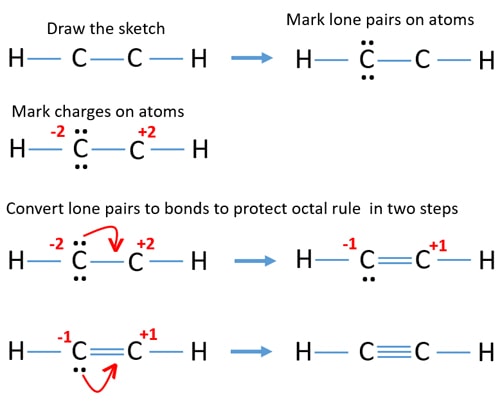
C2h2 Acetylene Ethyne Lewis Structure

C2h2 Molecular Geometry Shape And Bond Angles See Description For Note Youtube

C2h2 Lewis Dot Structure Geometry Youtube

C2h2 Lewis Structure Molecular Geometry Hybridization Polarity And Mo Diagram Techiescientist
C2h2 Lewis Structure Molecular Geometry Hybridization Bond Angle

C2h2 Acetylene Ethyne Lewis Structure

Lewis Structure For C2h2 Ethyne