Lewis Dot Structure Of Sef4
A step-by-step explanation of how to draw the SeF4 Lewis Dot Structure Selenium TetrafluorideFor the SeF4 structure use the periodic table to find the tot. Drawing the Lewis Structure for SeF 4.

Determine The Molecular Geometry Of Sef4 Clutch Prep
SeF 4 is Lewis structure with Selenium which can hold more than 8 valence electrons.

Lewis dot structure of sef4. Six plus twenty eight equals thirty-four total valence electrons. 1 1 Se 4 Br 6 47 34 valence electrons. This means that SeF4 has Trigonal Bipyramidal structure with 4 bond pairs and 1 lone Continue Reading You can guess the molecular structure of any molecule by knowing the no.
The Lewis structure helps one to understand the sharing of electrons between the central and neighboring atoms within a compound. Since there are seven Fluorine F atoms it will be necessary. Bond dipoles do not cancel.
The valence electrons that participate in forming bonds are called bonding pairs of electrons whereas the electrons that do not participate or form any. The bonds that are formed between two. Vapor is heavier than air.
Molar mass Cu 6355g mol. This gives Se four bond pairs and one lone pair. In the SeF 4 is Lewis structure Selenium Se is the least electronegative atom and goes in the center of the structure.
The bonds formed between two atoms are depicted using lines whereas the valence electrons not forming any bonds are shown by dots. The Lewis structure is like that of SeF₄. Similarly you may ask what is the Lewis structure for SeF4.
So lets multiply that out. SeF4 Lewis falicsempe konyha csempe ötletek Structure. Since there are seven Fluorine F atoms it will be necessary.
The way to determine the molecular geometry of SeF4 is to first draw the Lewis Dot Structure. Lewis structure is a pictorial representation of the bonds and valence electrons in the molecule. A step-by-step explanation of how to draw the AsO4 3- Lewis Dot StructureFor the AsO4 3- structure use the periodic table to find the total number of valenc.
Try to draw the xef 4 lewis structure before watching the video. Youll have a pair of electrons left over after filling octets of the F atoms. SeF4 is Lewis structure with Selenium which can hold more than 8 valence electrons.
Se on the periodic table is in Group Six so it has six valence electrons. Se must expand its octet. In the SeF 4 is Lewis structure Selenium Se is the least electronegative atom and goes in the center of the structure.
Here the central atom is Sulfur and the neighboring atoms are Fluorine. Since there are seven fluorine f atoms it will be necessary. Youll have a pair of electrons left over after filling octets of the F atoms.
SeF 4 is Lewis structure with Selenium which can hold more than 8 valence electrons. SF4 has a trigonal bipyramidal molecular geometry. Since there are seven Fluorine F atoms it will.
Fluorine seven valence electrons but we have four of those. How to Draw the Dot Structure. SF4 is a dangerous substance that is frequently utilized in chemical and pharmaceutical businesses.
You can put these on the central Se atom. The Lewis structure of SF4 is the combination of 34 valence electron and 5 electron pairs around the Sulfur in which there are four bonding pairs and one lone pair. Quiz your students on lewis dot diagram structure for sef4 molecular geometry hybridization.
Lets do the Lewis structure for SeF4 Lewis Structure. This electron arrangement is known as Trigonal Bipyramidal. This is the general idea of how and why Lewis structures are made.
Since there are seven Fluorine F atoms it will be necessary. There is one lone pair present on the central atom in the SeF4 lewis structure and 12 lone pairs on outer atoms. The shape is like a seesaw.
The electron arrangement for five electron pairs is trigonal bipyramidal. The central sulfur atom is linked to four fluorine atoms and contains one lone pair. SF4 lewis structure comprises one sulfur and four fluorine atoms.
SeF4 is Lewis structure with Selenium which can hold more than 8 valence electrons. Let us look at how SF4s Lewis structure can be formed. SeF4 is Lewis structure with Selenium which can hold more than 8 valence electrons.
SF4 Lewis Structure. SeF4 lewis structure is made up of one selenium and four fluorine atoms selenium is the central atom and fluorine is kept outside in the lewis diagram. A step-by-step explanation of how to draw the SF4 Lewis Dot Structure Sulfur tetrafluorideFor the SF4 structure use the periodic table to find the total n.

Draw The Lewis Structure Of Selenium Tetralfluoride Sef4 Clutch Prep

What Is The Molecular Geometry Of Sef4 How Is It Determined Quora

Sef4 Lewis Structure How To Draw The Lewis Structure For Sef4 Youtube
Is Sef4 Polar Or Nonpolar Quora

Write Lewis Structures For Sef4 And Sef6 Is The Octet Rule Satisfied For Se Study Com

File Selenium Tetrafluoride Svg Wikimedia Commons

Sf4 Lewis Structure How To Draw The Lewis Structure For Sf4 Youtube

Consider The Molecule Sef4 A Draw The Lewis Structure B What Is The Hybridization Of Se C What Is The Electron Geometry D What Is The Molecular Geometry E What Degree Angles
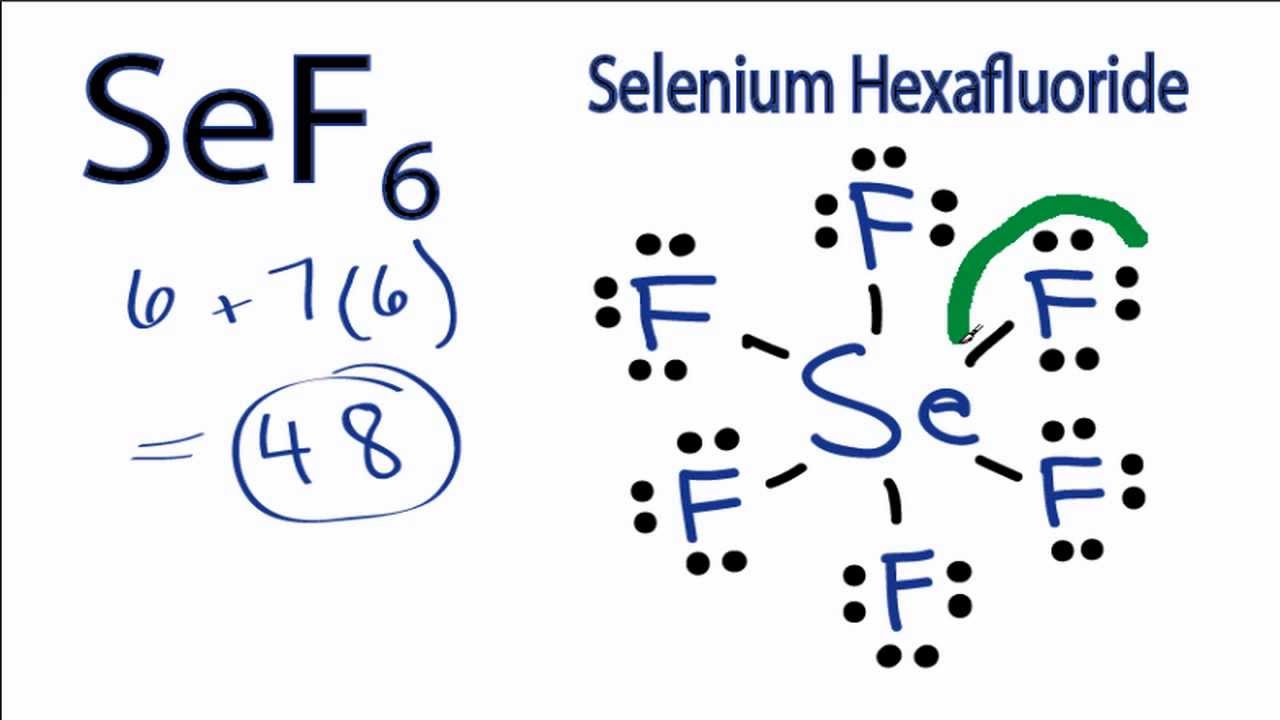
Sef6 Lewis Structure How To Draw The Lewis Structure For Selenium Hexafluoride Youtube

Sef4 Lewis Structure How To Draw The Lewis Structure For Sef4 Youtube

Sef4 Lewis Structure Molecular Geometry Polar Or Nonpolar Bond Angle

File Selenium Tetrafluoride Svg Wikimedia Commons
What Is The Molecular Geometry Of Sef4 How Is It Determined Quora

How Many Possible Fsef Bond Angles Are Present In Sef4 Molecule
Http Www Msubillings Edu Sciencefaculty Handouts Wiles Chem 20116 Solution 20set 202 Pdf
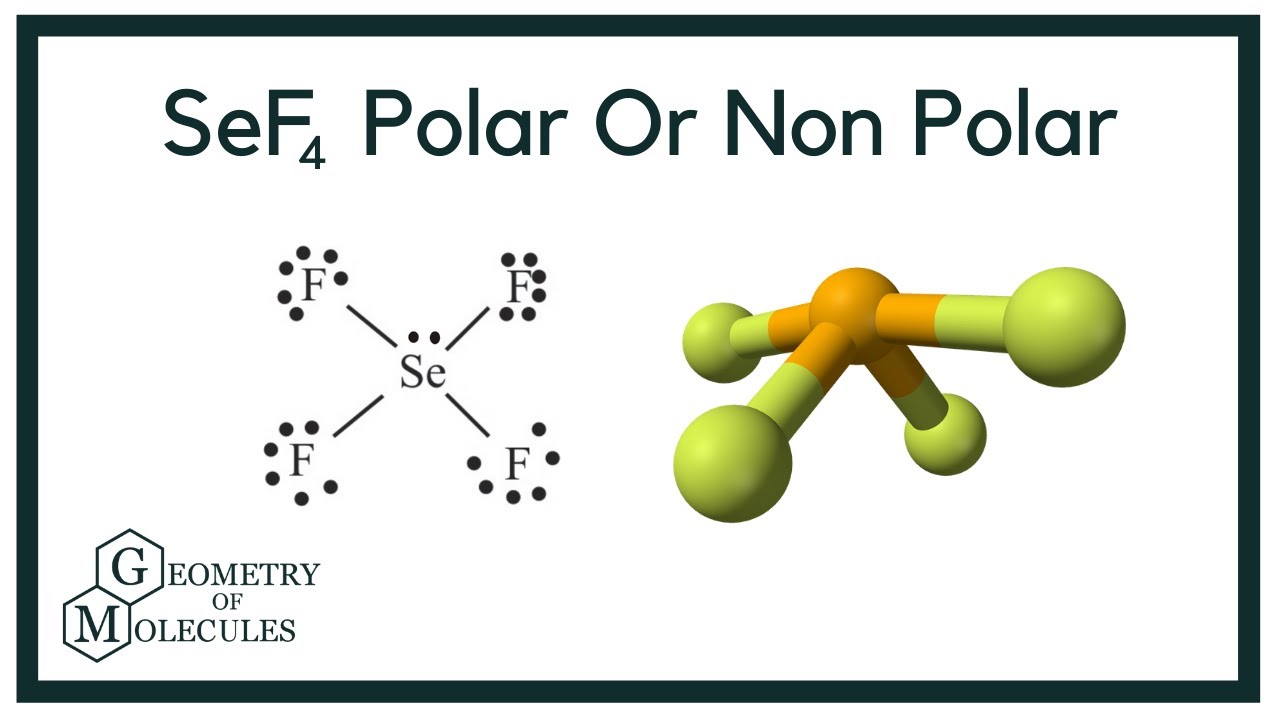
Is Sef4 Polar Or Non Polar Selenium Tetrafluoride Youtube
Sf4 Molecular Geometry Lewis Structure Bond Angles And Polarity

