Lewis Structure Of H2co2
November 18 2020 admin. Bonds and _____ double bonds.

Draw Lewis Structure Of Hcooh Formic Acid Youtube
A step-by-step explanation of how to draw the O2 Lewis Dot Structure Oxygen Gas Diatomic OxygenFor the O2 structure use the periodic table to find the t.

Lewis structure of h2co2. Chirality handedness of drugs. Hydrogen peroxide is polar in nature and has a non-planar structure. Lattice Energy Part II.
It has two oxygen atoms in the centre and they end up single-bonded. The bond angle of H2O2 in the gas phase is 948º and in the solid phase it is 1019º. I can tell you that the name IUPAC of formic acid is methanoic acid and his geometric arrangement is triangular with 120 among its three bonds.
Each oxygen atom has two pairs of free electrons. So there are two pairs of dots. Each element follows an octet rule in which it tries to attain a stable structure by having eight valence electrons in its outer shell.
Carbon C is the least electronegative atom and goes at the center of the H 2 CO Lewis structure. But c o x 2 cant accept electron pairs because oxygen and carbon both are electron sufficient have complete octets. Use information from step 4 and 5 to draw the lewis structure.
Alternatively a dot method can be used to draw the lewis structure. Carbon goes in the centreMake sure carbon and oxygen get 8 electrons to fulfil octet rule. Lewis structure of Hydrogen peroxide H 2 O 2 contains two O-H bonds and one O-O bond.
It has a linear bond geometry and no lone pairs. The correctly drawn Lewis dot structure for linear H2CO2 contains _____ lone pairs _____ single. For determining the Lewis structure remember the following points.
A step-by-step explanation of how to draw the C2H2 Lewis Dot Structure Ethyne or AcetyleneFor the C2H2 structure use the periodic table to find the total. The correct Lewis structure for a molecule of the compound C2H2 contains an alkyne bond. The Lewis dot structure of carbonic acid H2CO3 is as follows.
H2co2 lewis structure H2co. The hydrogen stations are located at. H2O2 Lewis Structure.
This is a triple bond between two C atoms. Fatty Acid Structures SaturatedUnsaturated. There are a total of 12 valence electrons in the H 2 CO Lewis structure.
A step-by-step explanation of how to draw the H2O Lewis Dot Structure WaterFor the H2O structure use the periodic table to find the total number of valenc. H2O2 molecular geometry is bent and electron geometry is tetrahedral. Note that the H2O2 Lewis structure is frequently used on tests a.
The total valence electron in H2O2 is 14. The Lewis dot structure for any molecule or compound helps determine the arrangement of atoms in the molecule bonds formed and lone pairs of electrons. Describe the orbitals used by each carbon atom in bonding and indicate the approximate bond angles1.
Molecular Geometry VSEPR. Two oxygen atoms are attached to a carbon atom by single coolant bonds. Asked Jun 26 2017 in Chemistry by SeaBunny.
Lattice Energy Part III. Also there are two lone pairs on each oxygen atom. A step-by-step explanation of how to draw the H2CO3 Lewis Dot Structure Carbonic AcidFor the H2CO3 structure use the periodic table to find the total numb.
Then there is a Hydrogen Atom at. A step-by-step explanation of how to draw the H2CO Lewis Structure Formaldehyde. Lewis dot structure of H 2 CO.
H 2 CO is also called Formaldehyde. Formaldehyde H2CO is the simpiliest of a class of functional groups call. Fill in the blanks with the appropriate words.
H 2 CO is the simpliest example of the organic functional group called the Aldehydes. The total lone pair present in the H2O2 lewis dot structure is 4. See below I never heard speak of geometric name and I are curious to know it.
A step by step explanation of how to draw the h2co lewis structure formaldehyde. Part 1 Born-Haber Cycle. One oxygen atom is bonded with the carbon atom by double covalent bond.
Calculate the total valence electrons in the molecule. Concept of number of total valence electrons of oxygen and hydrogen atoms are used to draw lewis structure of H 2 O 2. A step-by-step explanation of how to draw the H2O2 Lewis Dot Structure Hydrogen peroxide.
First would be the carbon with 3 regions of electron density so it would have a trigonal planar shape with bond angles of 120. In chemistry carbonic acid is a dibasic acid with the chemical formula h 2 co 3. Pin By Risitavidhya On Worksheets With Images Worksheets Personalized Items 10 Things Wyoming discount registered agent inc.
H2Co2 Lewis Structure. Each step of drawing lewis structure of H 2 O 2 is explained in detail in this tutorial. H2O2 hydrogen peroxide is a funny looking molecule.
The two hydrogen atoms are attached to each oxygen atom.

Lewis Structures Introduction Formal Charge Molecular Geometry Resonance Polar Or Nonpolar Youtube Molecular Geometry Molecular Chemistry

Ch3cl Lewis Structure Chloromethane In 2021 Molecules Lewis Methylation

Lewis Structure Math Equations Physical Science Chemistry

Ocn Lewis Structure How To Draw The Lewis Structure For Ocn Youtube

126 Hso4 Lewis Structure How To Draw The Lewis Structure For The Bisulfate Ion Youtube Science Chemistry Chemistry Organic Chemistry
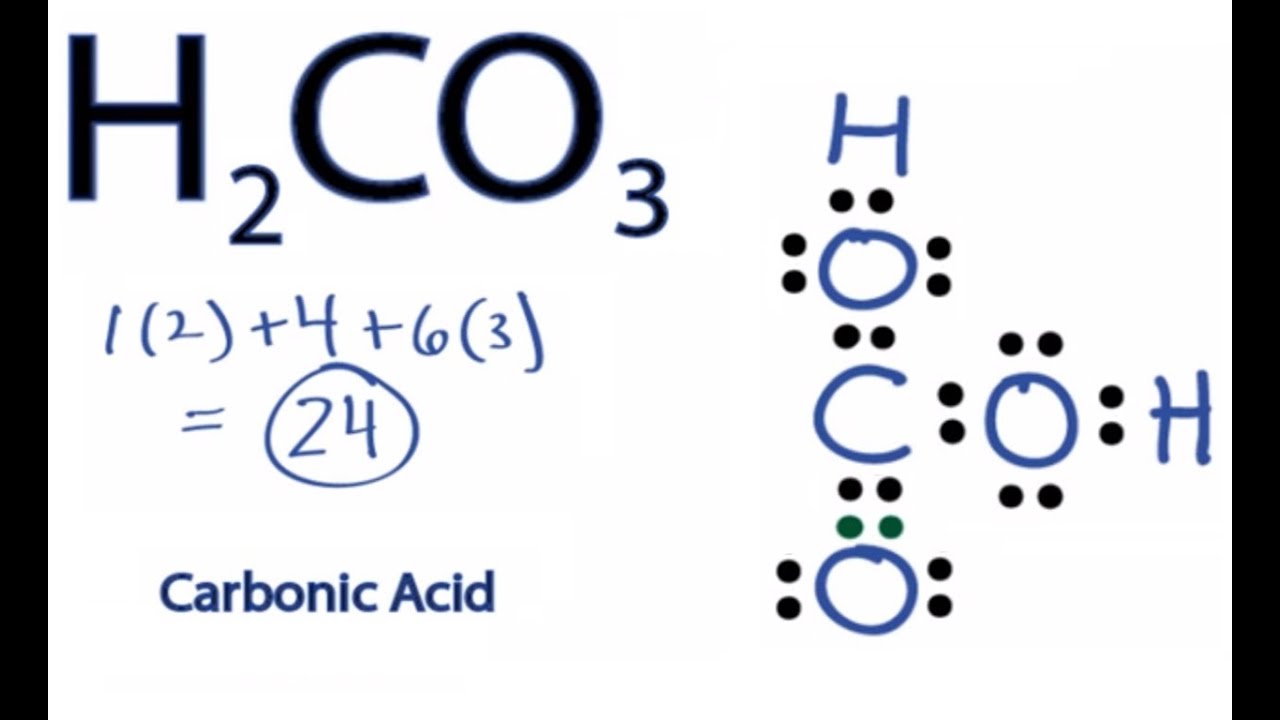
H2co3 Lewis Structure How To Draw The Lewis Structure For Carbonic Acid Youtube

Becl2 Lewis Structure Beryllium Chloride In 2021 Math Equations Lewis Molecules

Lewis Structure Of Hcooh Brainly In

Hcooh Lewis Structure How To Draw The Electron Dot Structure For Hcooh
What Is The Geometric Name For H2co2 Socratic

Sf4 Lewis Structure How To Draw The Lewis Structure For Sf4 Teaching Chemistry Chemistry Worksheets Chemistry Education

Draw A Lewis Structure Of Formaldehyde Lewis Chemistry Draw

Cl2co Lewis Structure How To Draw The Lewis Structure For Carbonyl Dichloride Youtube

Co2 Lewis Structure Carbon Dioxide Youtube

Cf2cl2 Lewis Structure How To Draw The Dot Structure For Cf2cl2 Dichl Drawings Dots Lewis

C2h2 Lewis Structure Ethyne Or Acetylene In 2021 Math Equations Lewis Molecules

Ch2o Lewis Structure Methanal Or Formaldehyde In 2021 Methanal Molecules Lewis

