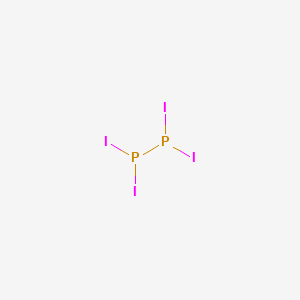
Lewis Structure P2i4
Diphosphorus tetraiodide is a chemical name of P2I4 P 2 I 4 in which two phosphorus are placed at the centre and the four iodide atoms surround the phosphorus atoms. Experiment 12 Lewis Dot Structures and Molecular Geometry 12-9 Name _____ Pre-Lab Assignment for Lewis Dot Structures and Molecular Geometry 1.
Select The Most Polar Bond Amongst The Following Chegg Com
Draw the Lewis structure for P2O4 including any resonance structures and resonance hybrids.

Lewis structure p2i4. The electronegativity of fluorine is greater than that of boronso the boron atom is placed in the center of the molecule. 5 4 3 2 1 Oxygen difluride is a powerful oxidizing and fluorinating agent. What is the limiting reagent in the above problem.
The Molecular Nature of Matter and Change 6th Edition Edit edition Solutions for Chapter 10 Problem 10MCQ. A 1 megagram 106 gram b 1 milliliter 10-3 liter and 12. It is the most stable of the diphosphorus tetrahalides.
Select the best Lewis structure for P2I4. Draw the Lewis structure for boron trifluoride BF 3. Draw a skeleton for the molecule which connects all atoms using only single bonds.
Diphosphorus tetraiodide is an orange crystalline solid with the formula P2I4. The total number of electron is 24 37 from each fluorine 3 from boron 24. Using a single bond between the boron and each of the fluorine atoms.
Get the detailed answer. Select its Lewis structure. The XeO2F2 Lewis structure is a good structure to help you understand why calculating f.
How many electron pairs are shared between the carbon atoms in C_2H_4. Select the best Lewis structure for P2I4ABCDE None of the above structures is suitable for P2I4. In simple molecules the atom with the most available sites for bondng is usually placed central.
What is the chemical name for p2i4. The formula for diphosphorus trioxide is P2 O3. A molecule with the formula AX4 uses _____ to form its bonds.
That gives us a total 14 valence electrons for the P2H4 Lewis structure. Click to see full answer. Select the best Lewis structure for P2I4 predict the electron group arrangement of each central atom and the shape of this molecule.
Free unlimited access for 30 days limited time only. Write the number of valence electrons for each atom total number of valence. Start your trial now.
Hydrogen atoms always go on the outside of Lewis structures so were goint to put the two phosphorus atoms in. A step-by-step explanation of how to draw the XeO2F2 Lewis Structure. A P P I I I I.
The amount of chloride ion in a water supply can be determined by reaction with aqueous silver nitrate. Chapter 1 Exercises 11. 1 N 2 O 5 2 N 2 3 O 2 4 both N 2 and O 2 5 none of these 8.
Determine the total number of valence electrons in a molecule 2. How many valence electrons are shown in the Lewis structure of NO 3 A 11 B 15 C from SCIE 131 at University of British Columbia. Select the best Lewis structure for P_2I_4.
Yes or No Overall Molecular Polarity Polar or Nonpolar. Any Polar Bonds in Molecule. 230 g because the reported values differ by about 001.
For the P2H4 Lewis structure Phosphorous is in Group 5 or 15 is has five valence electrons but we have Phosphorus atoms a nd then we have Hydrogen with one valence electyron but we have four hydrogen atoms. What is the best Lewis structure for P 2 I 4. It has been used as a reducing agent in organic chemistry.
It is a covalent compound and. Loose Leaf Version for Chemistry. For every resonance hybrid assign the bond order.
It is a rare example of a compound with phosphorus in the 2 oxidation state and can be classified as a subhalide of phosphorus. Thionyl chloride is used as an oxidizing and chlorinating agent in organic chemistry. Log in Sign up.
What is the best Lewis structure for P2I4. Chapter 1 Key Ideas 1. Observation data hypothesis research or experimentation research published applications hypothesizing and testing 3.
An outline of how to detemine the best Lewis structure for an example NO 3-is given below. A 71 mL to 73 mL b 822 m to 824 m c 454 10-5 g to 456 10-5 g 13. According to VSEPR theory a molecule with general formula AX4E will have a _____ molecular shape.
Select of the best lewis structure for P2I4i. Just so what is the formula for diphosphorus tetroxide. A P P I I I I B P P I I I I C P P I I I I D P P I I I I E None of the above structures is suitable for P2I4.
None of the above structures is suitable for P_2I_4.

Chapter10q 1 Chapter 10 The Shapes Of Molecules Which One Of The Following Lewis Structures Is Definitely Incorrect 5 Hydrazine N2h4 Is A Good Course Hero
Select The Best Lewis Structure For P 2i 4 None Of Chegg Com
Preparation And Structures Of 1 2 Dihydro 1 2 Diphosphaacenaphthylenes And Rigid Backbone Stabilized Triphosphenium Cation Dalton Transactions Rsc Publishing Doi 10 1039 B517071k

Infrared And Raman Spectra Of Inorganic And Coordination Compounds 6 Ed Part A Nakamoto Pages 201 250 Flip Pdf Download Fliphtml5

Oneclass What Is The Best Lewis Structure For P2i4
Chem 1301 Second Test Review Lewis Structures Flip Ebook Pages 1 8 Anyflip Anyflip
Select The Most Polar Bond Amongst The Following Chegg Com
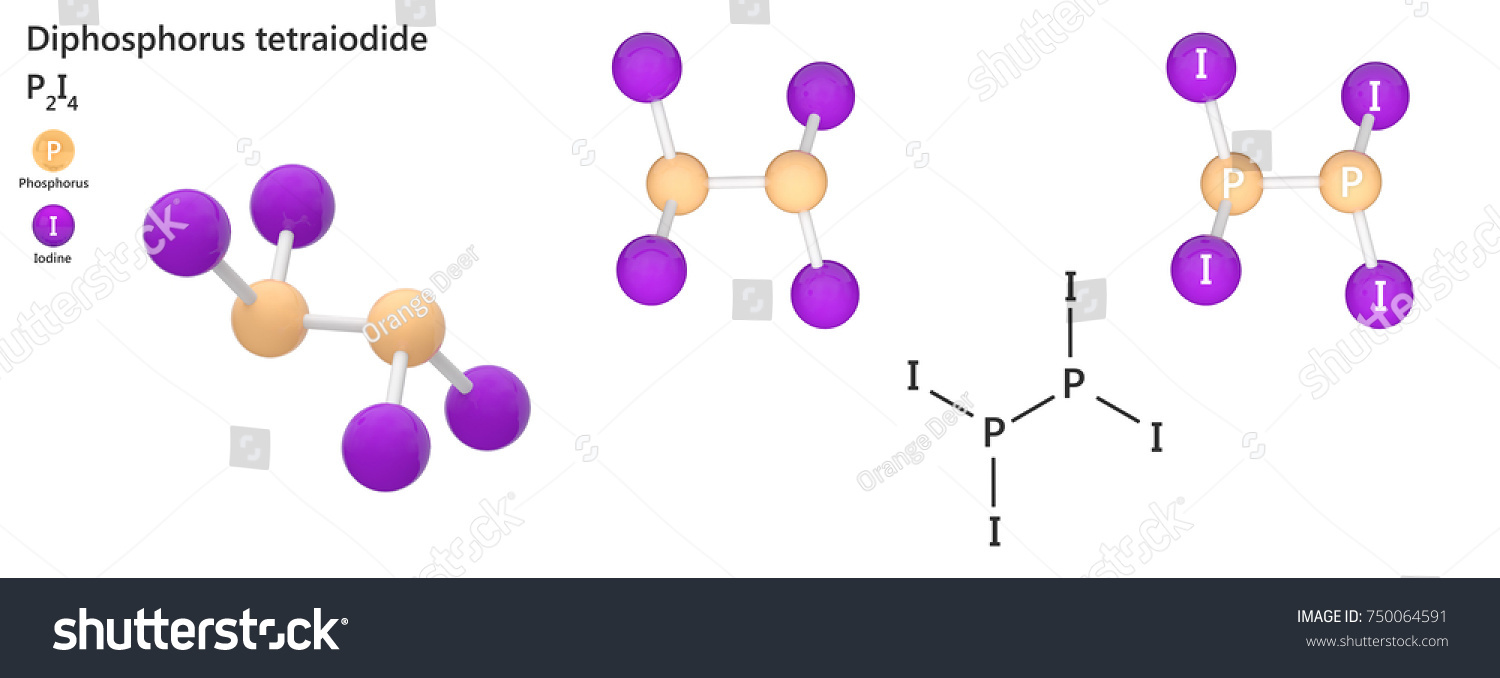
Diphosphorus Tetraiodide Orange Crystalline Solid Formula Stock Illustration 750064591
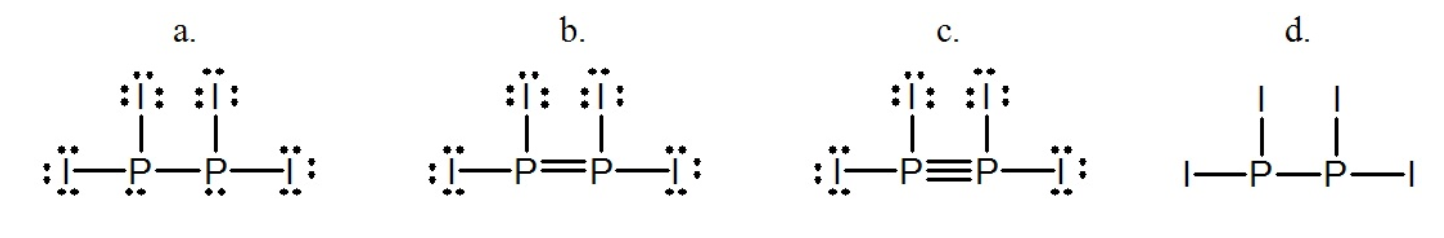
Answered Select The Best Lewis Structure For Bartleby
Hypodiphosphorous Tetraiodide P2i4 Pubchem
Chem 1301 Second Test Review Lewis Structures Flip Ebook Pages 1 8 Anyflip Anyflip

Oneclass What Is The Best Lewis Structure For P2i4

P2h4 Lewis Structure How To Draw The Lewis Structure For P2h4 Youtube

Lab Final All Flashcards Quizlet
Chem 1301 Second Test Review Lewis Structures Flip Ebook Pages 1 8 Anyflip Anyflip

Oneclass What Is The Best Lewis Structure For P2i4
Chem 1301 Second Test Review Lewis Structures Flip Ebook Pages 1 8 Anyflip Anyflip
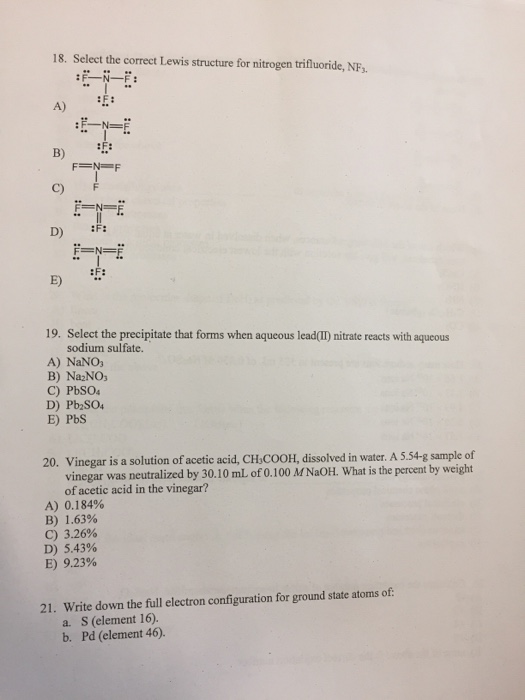
18 Select The Correct Lewis Structure For Nitrogen Chegg Com