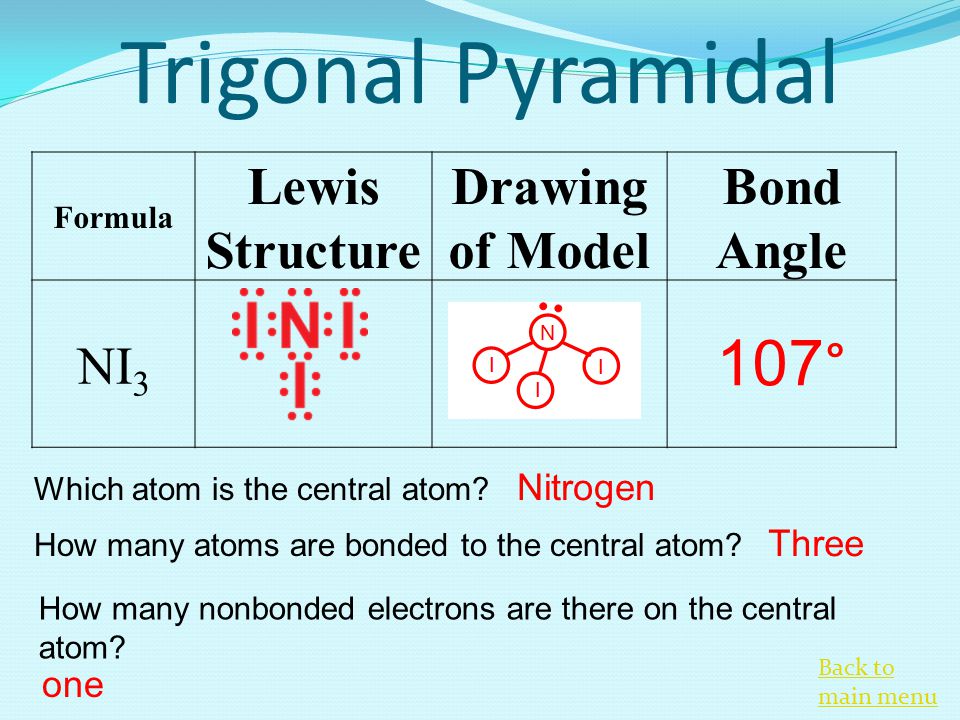
Ni3 Vsepr Structure
There are lone pairs on X or other atoms but we dont care. Count its valence electrons 3.
It can even be detonated by alpha radiation.
Ni3 vsepr structure. The Xenon atom has 4 bonding pairs of electrons and 2 lone non-bonding pairs of electro. What is their structure. Add or subtract electrons for charge see Top Tip 5.
Add one electron for each bonding atom 4. A step-by-step explanation of how to draw the NO3- Lewis Structure Nitrate Ion. VSEPR Theory Practice Problems.
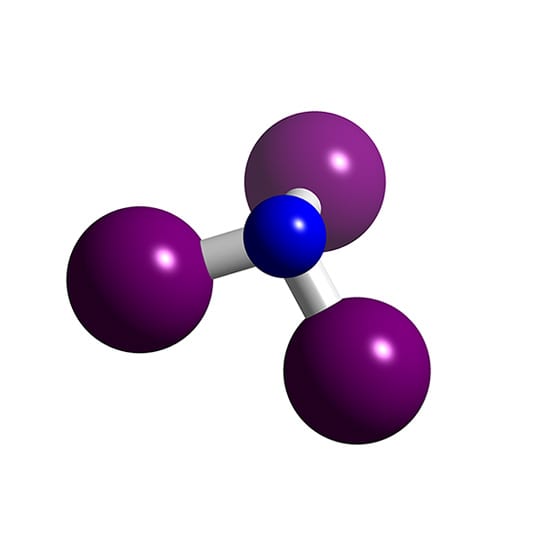
VSEPR Theory Molecular Shapes A the central atom X an atom bonded to A E a lone pair on A Note. NI3 has a complex structural chemistry that is difficult to study because of the instability of the derivatives. In all these compounds the nitrogen atom has a complete octet with four outer-shell electron pairs.
Xenon tetrafluoride XeF4 is a square planar non-polar molecule. The molecular geometry or three-dimensional shape of a molecule or polyatomic ion can be determined using valence-shell electron-pair repulsion abbreviated VSEPR and pronounced VES-per theory in. Total Domains Generic Formula Picture Bonded Atoms Lone Pairs Molecular Shape Electron Geometry.
The total electron pairs bonding pairs and non-bonding pairs are tabulated on the same screen. It is an extremely sensitive contact explosive. The structure of I3 is bent or V-shaped and the hybridization is sp3.
These arrange themselves as far apart as possible around the nitrogen atom in a roughly tetrahedral disposition to minimise repulsions between the negative charge clouds. Select the correct code for the following repulsion orders according to VSEPR theory I lone pair-lone pair lone pair-bond pair II lone pair-bond pair bond pair-bond pair III lone pair-lone pair bond. However because repulsions involving lone pairs.
From these three set of numbers the molecular geometry is determined. VSEPR Model VALENCE-SHELL ELECTRON-PAIR REPULSION VSEPR MODEL Lewis structures show the two-dimensional distribution of atoms and electrons. The VSEPR model can predict the structure of nearly any molecule or polyatomic ion in which the central atom is a nonmetal as well as the structures of many molecules and polyatomic ions with a central metal atom.
Number of lone pairs on the N in NI3. In the case of NO3- the trigonal planar geometry is found. According tp VSEPR theory predict the geometry and shape of i3 ion.
12 Noof valence electron in central atom Monovalent atom attached to central metal atomanionic charge-cationic charge Applying 12 72-10 4 sp3. One of these is a non-bonding lone pair. Divide the total of these by 2 to find the total number of electron pairs.
Small quantities explode with a loud sharp snap when touched even lightly releasing a purple cloud of iodine vapor. A step-by-step explanation of how to draw the I3 - Lewis Dot Structure Triiodide IonFor the I3 - structure use the periodic table to find the total number. If playback doesnt begin shortly try restarting your device.
Electron pairs gives base shape Octahedral VSEPR base shape for 6 e-pairs Refcode. The premise of the VSEPR theory is that electron pairs located in bonds and lone pairs repel each other and will therefore adopt the geometry that places electron pairs as far apart. What does VSEPR stand for.
The VSEPR structure of the input molecule is displayed along with the name of the geometrical structure. We are interested in only the electron densities or domains around atom A. Ni3 lewis structure shape To a first approximation the VSEPR model assumes that multiple bonds and single bonds have the same effect on electron pair geometry and molecular geometry.
In other words VSEPR treats multiple bonds like single bonds. Only when considering fine points of molecular structure does VSEPR recognize that multiple bonds occupy more space around the central atom than. Nitrogen triiodide is an inorganic compound with the formula NI3.
Identify the central atom 2. The formula for determining the hybridization is.
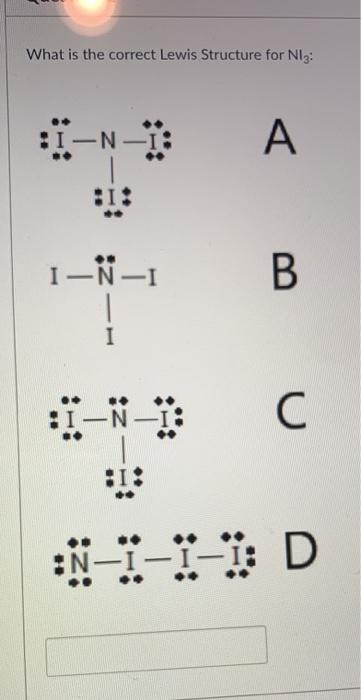
Draw The Lewis Structure For Each Species Below Then Chegg Com

How To Draw Lewis Structure Of Ni3 Drawing Easy

Oneclass What Is The Lewis Structure Of Ni3
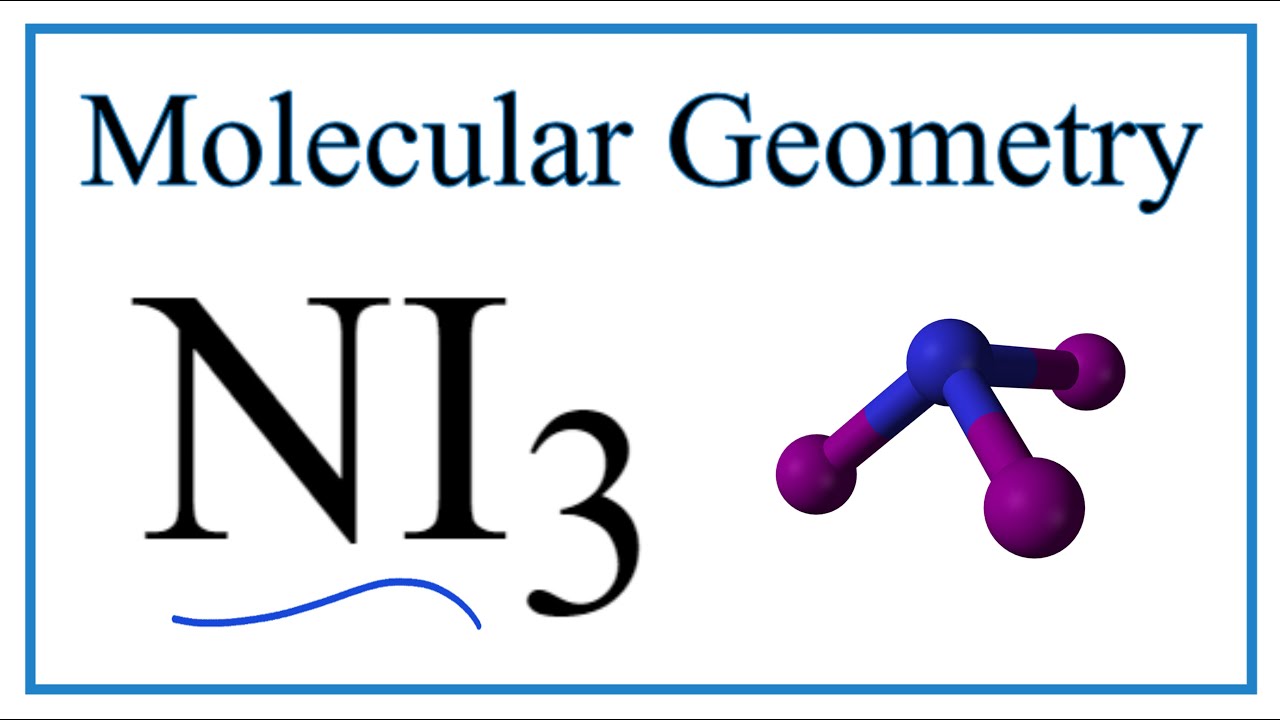
Ni3 Molecular Geometry Bond Angles Electron Geometry Youtube

What Is The Lewis Structure Of Ni3 Study Com

Molecular Models Activity Carbon Tetrachloride Ammonia Methane Hydrogen
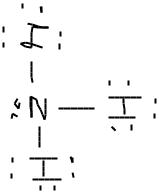
I Believe The Lewis Structure Looks Like This For Chegg Com

The Vsepr Theory By Eunice Yeaineedalife
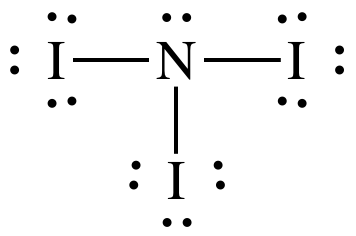
Covalent Bonding And Nomenclature Ppt Download
Ni3 Molecular Geometry Learn Lif Co Id
What Is The Correct Lewis Structure For Ni3 I N A Chegg Com

Ni3 Lewis Structure How To Draw The Dot Structure For Ni3 Nitrogen Triiodide Youtube
When A Covalent Lewis Structure Is Drawing Using One Nitrogen N How Many Lodine Atoms Will Bond To The Nitrogen Quora
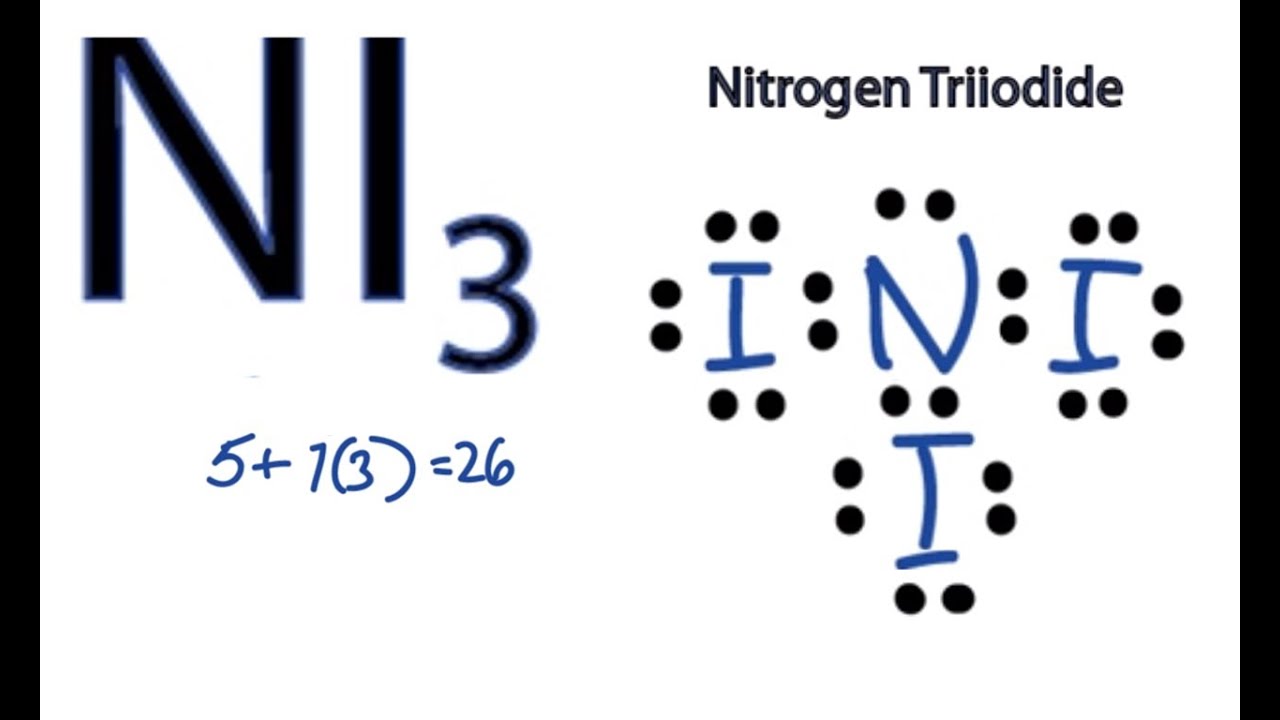
Ni3 Lewis Structure How To Draw The Dot Structure For Ni3 Nitrogen Triiodide Youtube

File Nitrogen Iodide 2d Png Wikipedia