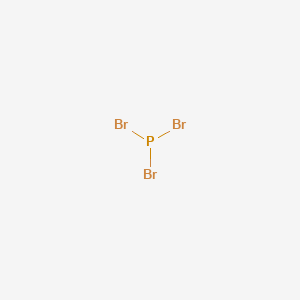Pbr3 Acid Or Base
The electronegativity of Br is 296 and that of P is 216. These protons go on to.
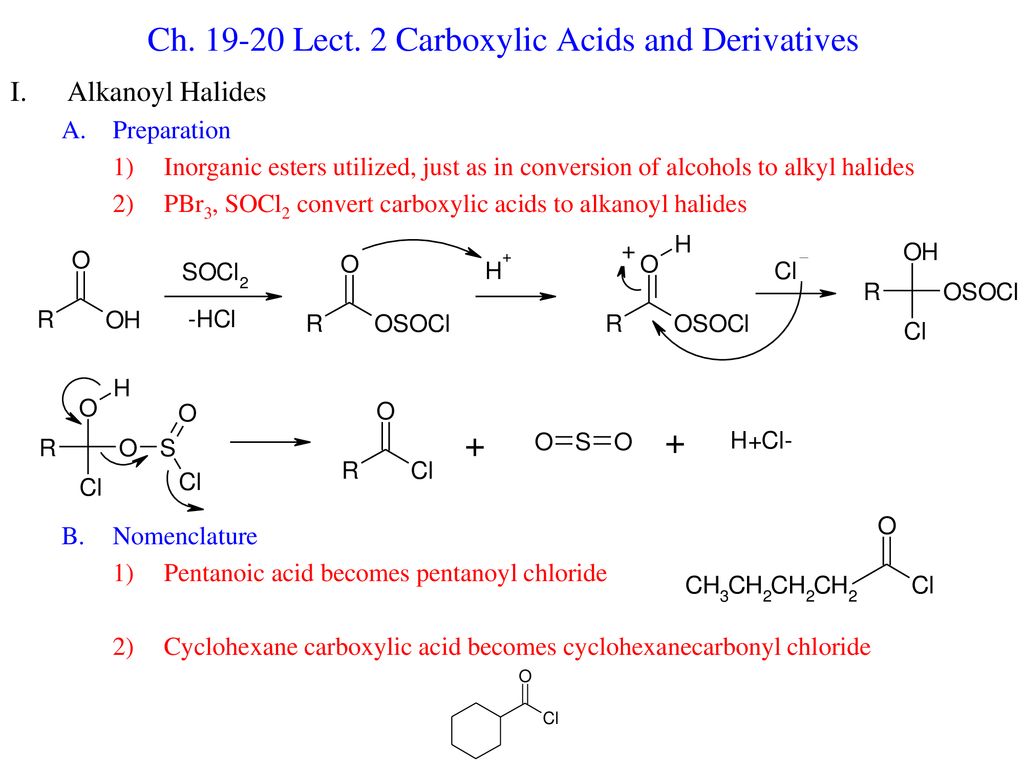
Ch Lect 2 Carboxylic Acids And Derivatives Ppt Download
Boiling Point C Clonality.
Pbr3 acid or base. NF 3 NCl 3 NBr 3 NI 3. Phosphorus tribromide like PCl 3 and PF 3 has both properties of a Lewis base and a Lewis acid. Since the exposure limit for HBr.
So Is PBr3 polar or nonpolar. Lewis acids have a tendency to accept electrons. List molecules Acid and Base.
An acid is a substance molecule or ion which can accept a pair of electrons. At the same time PBr 3 can react as an electrophile or Lewis acid in. Since iodone is least electronegative it is the most basic trihalide of nitrogen.
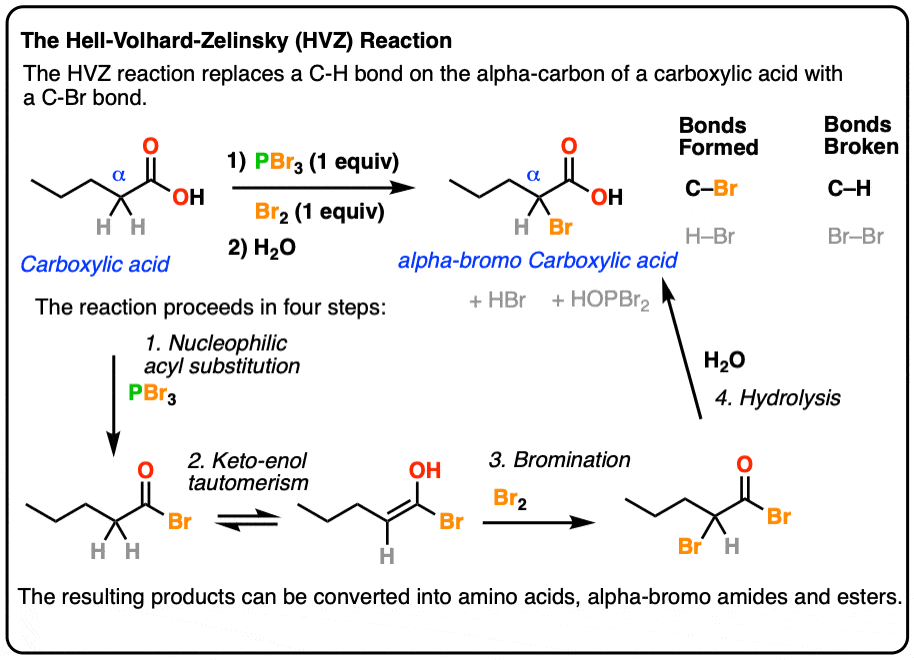
P can expand its octet due to presence of vacant d -orbitals. Optically active carbonyl compounds of the type ce-CHCO in which the alpha carbon is asymmetric are racemized by both acids and bases and from Section 17-1 we can be sure that this is related to enolization. Draw a plausible mechanism for the reaction of propionic acid with phosphorus tribromide to synthesize propanoyl bromide an acid bromide.
The sequence for the Lewis acid strength for. Phosphorus tribromide like PCl 3 and PF 3 has both properties of a Lewis base and a Lewis acid. For example with a Lewis acid such as boron tribromide it forms stable 1 1 adducts such as Br 3 B PBr 3.
Phosphorus tribromide like PCl 3 and PF 3 has both properties of a Lewis base and a Lewis acid. Is PBr3 Acid or Base. If you want to quickly find the word you want to search use Ctrl F then type the word you want to search.
Primary antibodies 3 antibodies 2 plasmids 1 Brand. Keep these important points in mind while guessing Lewis acids or bases. SO we have the trend in decreasing order of basic strength.
The P B r bond is polar due to the electronegativity difference between the atoms and hence P develops partial positive charge favouring a nucleophilic attack on it. Applications Products Services Support. Phosphorus tribromide reacts with moisture in the air to produce phosphonic acid and hydrogen bromide gas HBr.
Sigma-Aldrich 10 Biological Source. Which pair is a BrønstedLowry conjugate acidbase pair. Is FeBr3 an acid or base or salt.
A 10 cc cartridge of PBr3 tested as fire suppressant would react to form an estimate 25 g of HBr. PBr3 phosphorus tribromide is a polar molecule because of its asymmetrical shape. Formation of either the enol or the enolate anion will destroy the asymmetry of the alpha carbon so that even if only trace amounts of enol are present at any given time eventually.
Base is a substance which can donate a pair of electrons to form a co-ordiante covalent bond. Microsoft Word - Sn2 Substitution of Primary Alcohol with PBr3 Created Date. H Ions HCl BF3 etc.
If uniformly distributed in a room 10X10X5 m in size the maximum HBr concentration would be 005 mgL 50 mgcu inch. Whether a molecule eg. Then add something like 500mg of boron tribromide.
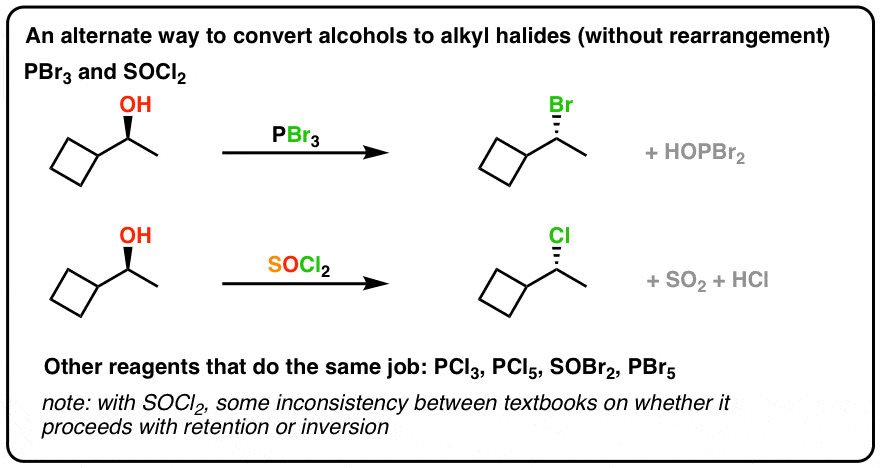
The main use for phosphorus tribromide is for conversion of primary or secondary alcohols to alkyl bromides as described above. ClO2 Bronsted Base Base. FeBr3 is salt What is an acid base salt.
For example with a Lewis acid such as boron tribromide it forms stable 1 1 adducts such as Br 3 B PBr 3. It will be helpful to draw Lewis structures. The why of a mechanism is always the best approach.
In the following neutralization reactions classify each reactant as a Lewis acid or base. PF 3 PCl 3 PBr 3 PI 3. Ships Today 5 Product Category.
But due to its asymmetrical shape it is considered a polar. At the same time PBr 3 can react as an electrophile or Lewis acid in. Lewis Concept of Acid and Bases.
As mentioned above phosphorous has empty d orbital in its valence shell to accept. ROR BBr3 ROBBr2 RBr ROBBr2 3 H2O ROH BOH3 2 HBr The reaction is thought to involve attack by a bromide ion on the Lewis acidbase adduct of the ether with BBr3 a strong Lewis acid. Ill tell you the Acid or Base list below.
It would be nonpolar covalent because the electronegativity. Find pbr3 and related products for scientific research at MilliporeSigma. What type of bond is PBr3 and PBr3.
Hence the electronegativity difference is greater than 05 making the P-Br bonds polar. Almost all simple cations which have an empty orbital in their outermost shell accept a pair of electrons from other molecules and function as Lewis acids. Phosphorus tribromide can be used both as a Lewis base and Lewis acid making it quite useful for various reactions.
If in a 615X462X148 m airplane hanger it would be 59X10-4 mg L 059 mgcu inch. Lewis base gives out a lone pair of electronsN of NH3 has a lone pairSo it can act as Lewis base. PBr3 is lewis Base.
Is PBr3 an acid or base. For example with a Lewis acid such as boron tribromide it forms stable 11 adducts such as Br 3 B-PBr 3. PBr3 usually gives higher yields than hydrobromic acid and it avoids problems of carbocation rearrangement- for example even neopentyl bromide can be made from the alcohol in 60 yield.

Reaction Of Alcohols With Pbr3 Via Sn2 Mechanism Organic Chemistry Chemistry Alcohol

The Hell Volhard Zelinsky Reaction Master Organic Chemistry

Substitution With Pbr3 Socl2 Video Lecture Chad S Prep
Pbr3 Lewis Structure Molecular Geometry Hybridization And Polarity

Is Pbr3 A Lewis Acid Or Base Or Neutral
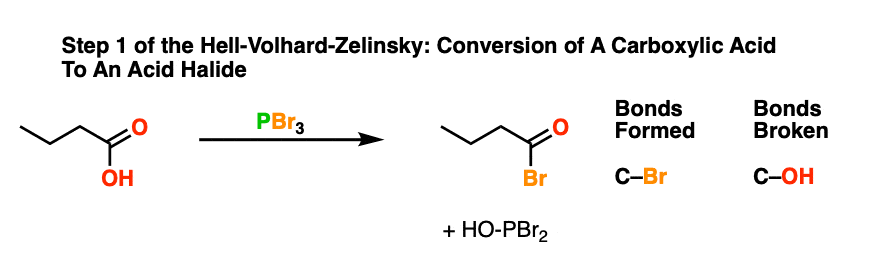
The Hell Volhard Zelinsky Reaction Master Organic Chemistry

Pbr3 Lewis Structure Molecular Geometry Polarity And Hybridization Techiescientist
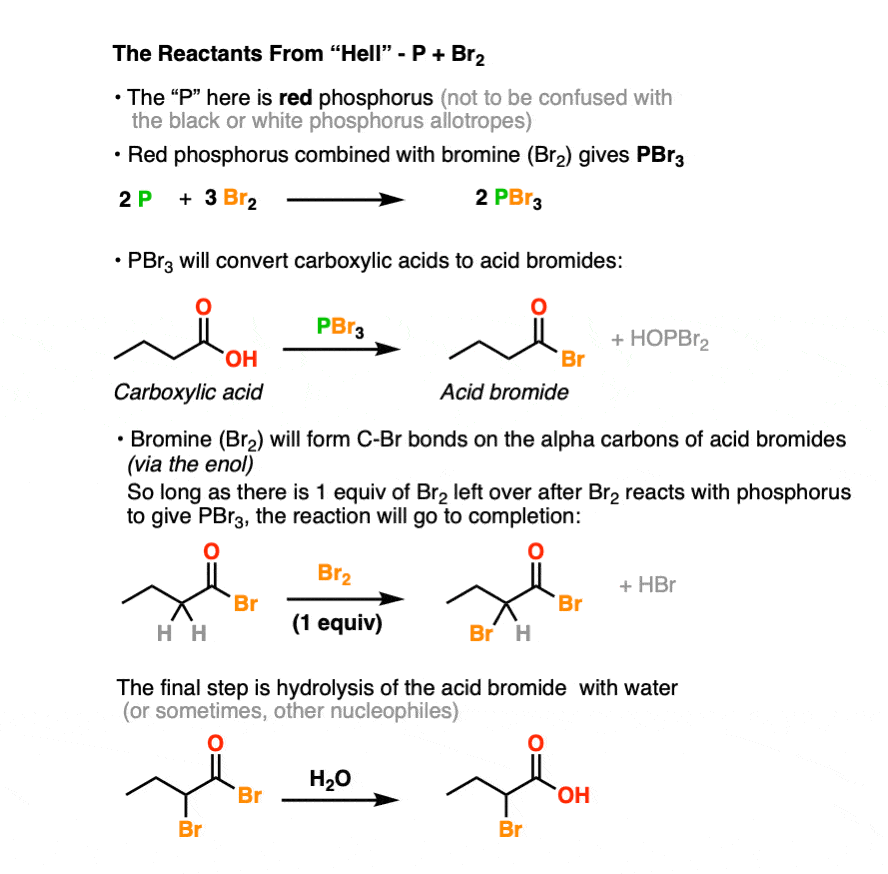
The Hell Volhard Zelinsky Reaction Master Organic Chemistry
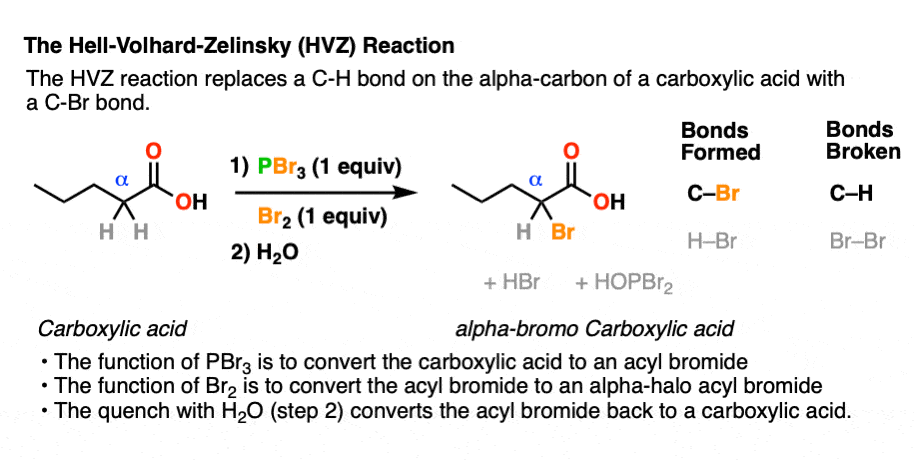
The Hell Volhard Zelinsky Reaction Master Organic Chemistry

Pbr3 Lewis Structure Molecular Geometry Polarity And Hybridization Techiescientist
Phosphorus Tribromide Pbr3 Pubchem

Substitution With Pbr3 Socl2 Video Lecture Chad S Prep

Hell Volhard Zelinski Reaction
Chemical Forums Precise Mechanism Of Hell Volhard Zelinsky Halogenation
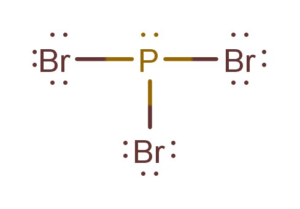
Pbr3 Lewis Structure Molecular Geometry Hybridization And Polarity

Why Does Phosphorus Tribromide Act As A Lewis Acid Electron Acceptor Chemistry Stack Exchange
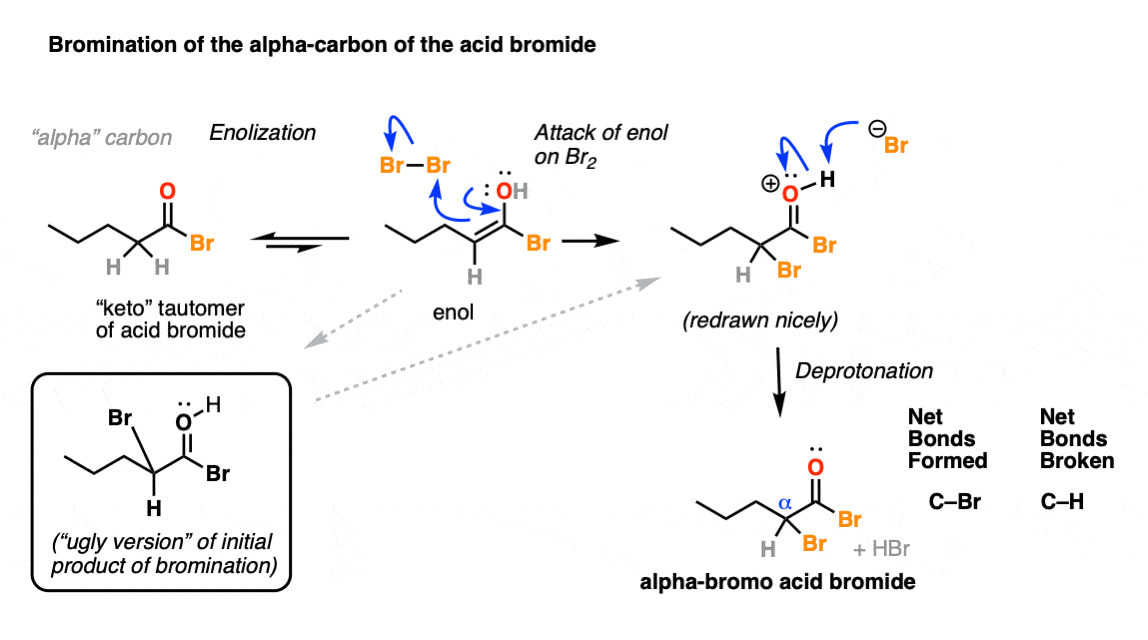
The Hell Volhard Zelinsky Reaction Master Organic Chemistry
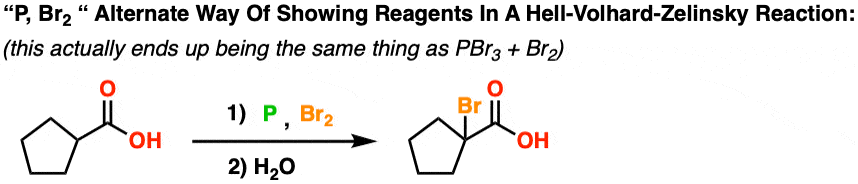
The Hell Volhard Zelinsky Reaction Master Organic Chemistry