Pcl5 Lewis Structure Octet Rule
A step-by-step explanation of how to draw the PCl4 Lewis Dot StructureFor the PCl4 structure use the periodic table to find the total number of valence el. The molecule has 14 valence electrons.
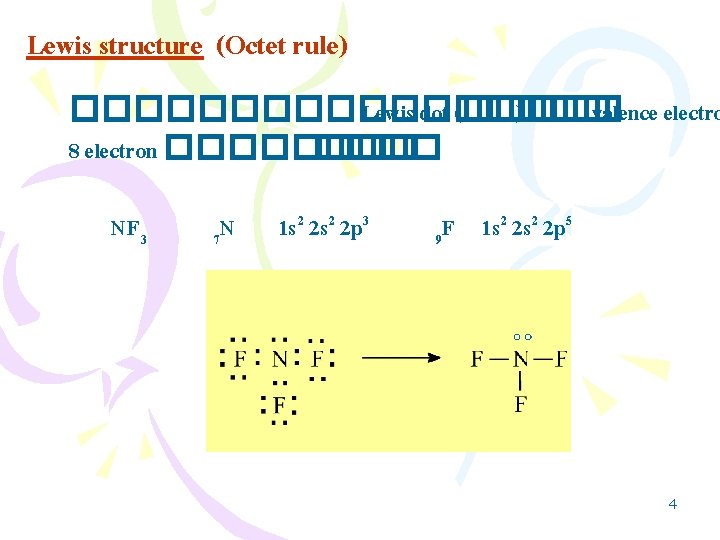
3 Lewis Structure Octet Rule Lewis Dot Valence
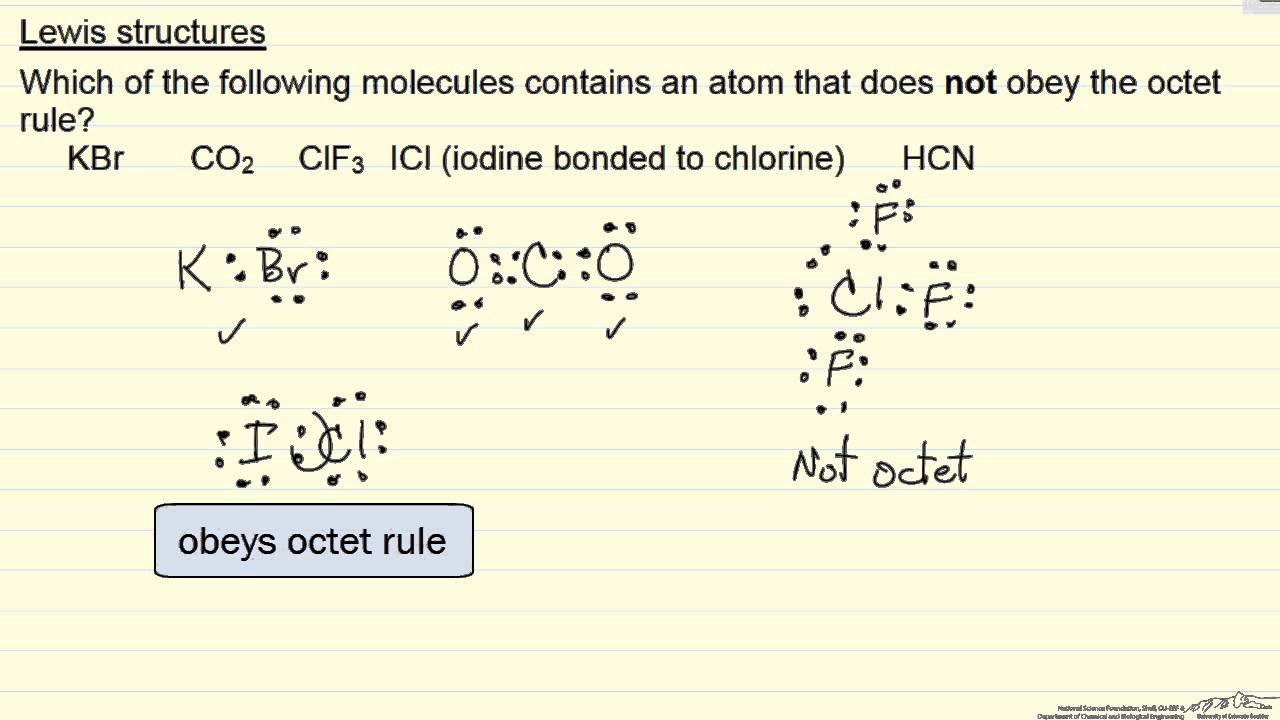
Most structuresespecially those containing second row elementsobey the octet rule in which every atom except H is surrounded by eight electrons.
Pcl5 lewis structure octet rule. ReadDownload File Report Abuse. In the PCl 5 molecule the central phosphorus atom is bonded to five Cl atoms thus having 10 bonding electrons and violating the octet rule. If you calculate the formal charge for each atom you will notice that fluorine which is the most electronegative element has a positive formal charge.
Why can Phosphorus have 5 bonds instead of 4. In an exam how would we now if this applies the octet rule. Postby Julie Nguyen 1B Mon Jul 11 2016 728 am.
How does PCl3 obey octet rule. However Lewis rules predict that Beryllium should rather form double bonds in order to have an octet. There are four bonding electron pairs around.
If all the phosphorus-chlorine bonds in a PCl 5 molecule are covalent it would imply that the phosphorus molecule is violating the octet rule by holding a total of 10 valence electrons. 5 Is PCl5 dipole dipole. For example P C l 5 is a legitimate compound whereas N C l 5 is not.
Lone pairs unpaired electrons and single double or triple bonds are used to indicate where the valence electrons are located around each atom in a Lewis structure. Exceptions to the octet rule occur for odd-electron molecules free radicals electron. Expanded valence shells are observed only for elements in period 3 ie.
4 Is PCl3 a Lewis structure. Lewis Dot of Phosphorous Pentachloride. 8 Is CCl4 a hydrogen bond.
The Octet rule is almost exclusivley used for elements with unfilled S and P orpitals with the implication being the compounds that are most stable have filled up the S 2 electrons and P 6 ekectrons orbitals. Molecules such as NO NO 2 and ClO 2 require a more sophisticated treatment of bonding which will be developed in Chapter6. The formation of five bonds by the phosphorus molecules can be explained by the sp 3 d hybridization in PCl 5.
But there are only certain places they can orbit. Phosphorous having valence electrons in the 3rd energy level will also have access to the 3d sublevel thus allowing for more than 8 electrons. 12 What is the weakest to strongest intermolecular force.
Think of orbitals like this. It will hold more than 8 electrons. Phosphorus pentachloride PCl5 as exception to octet rule.
We got phosphorus trichloride and YES this. Lavelle drew the Lewis structure for phosphorus pentachloride PCl5 and 5 chlorine atoms were attached to one phosphorus. To understand this we need to look at the electron configuration of a phosphorus atom which.
The overall geometry of the molecule is depicted trigonal bipyramidal and bond angles and lengths are highlighted. The Octet Rule is really more of a rule of thumb - and one particularly for organic chemistry at that - than a rule rule. With 5 6 11 valence electrons there is no way to draw a Lewis structure that gives each atom an octet of electrons.
As important and useful as the octet rule is in chemical bonding there are some well-known violations. Does H2O follow the octet rule. In a molecule of phosphorus pentachloride P Cl5 each chlorine atom ends up with an octet of electrons four pairs but the phosphorus atom ends up with ten electrons five pairs.
11 How many angstroms is a hydrogen bond. In the PCl5 molecule the central phosphorus atom is bonded to five Cl atoms thus having 10 bonding electrons and violating the octet rule. 1 PCl5 P has 5 valence electrons.
Does PCl5 follow the octet rule. Even without such a treatment we notice that such odd electron species are extremely reactive. Electrons being negative try to do two things stay away from each other and like most people use as little energy as possible.
7 Why does CCl4 have no dipole moment. Drawing the Lewis structure of PCl5. P does not follow the octet rule.
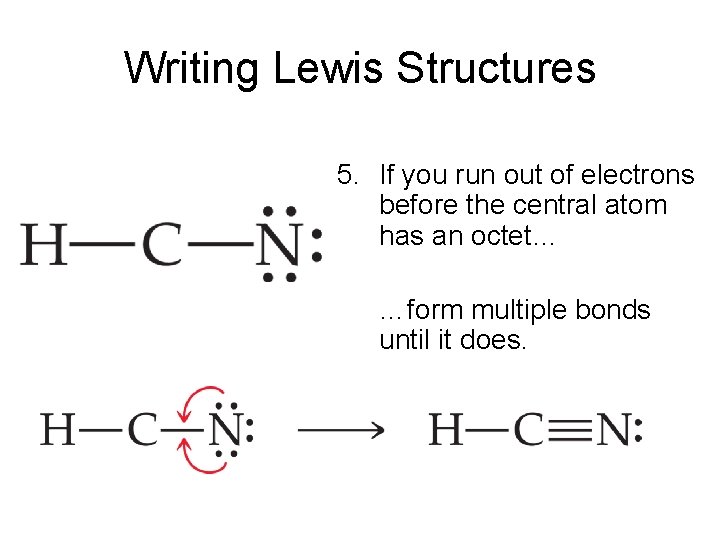
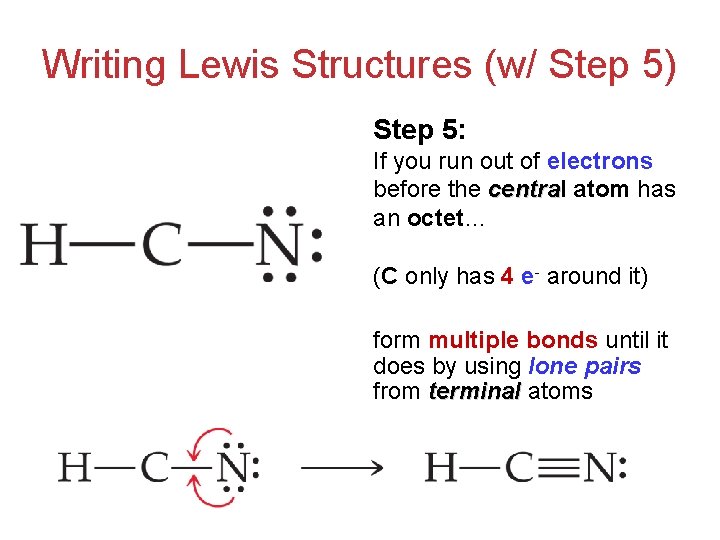
Why does PCl5 break the Octet Rule. Draw a Lewis dot structure for the formaldehyde molecule CH2O. Those atoms can be the same element as when oxygen bonds with itself to form O2 or with different elements such as water H2O.
So only the octet of oxygen. The P in PCl 5 has 10 valence electrons. Lewis structure with formal charges.
70 More Lewis Dot Structures. When drawing the Lewis structure for PCl5 five chlorine Cl atoms are bonded to the central atom phosphorous P. 9 Is co2 a dipole.
This chemistry video tutorial discusses the exceptions to the octet rule while providing the lewis dot diagrams of the molecular compounds involved. The octet rule is based upon available n s and n p orbitals for valence electrons 2 electrons in the s orbitals and 6 in the p orbitals. 10 Why cant CCl4 be hydrolysed.
ReadDownload File Report Abuse. When could the octet rule be broken. D How many bonding pairs are in the molecule.
Here are the double bonds. The number of sigma bonds in a molecule is always give by the number of single bonds between the atoms -- PCl5 has 5 sigma bonds and water has 2 if a molecule possesses one double bond then the count goes to 1 sigma bond and a 1 pi bond for a triple bond - 1 sigma bond and 2 pi bonds. Post by AJ Manzano 3K Sun Nov 04 2018 1049 pm.
In Wednesdays lecture Dr. 6 Does CCl4 have a dipole moment. Chapter 3 - Molecular Shape and Structure 1 The Lewis structure of CH3F C is the central atom is.
The chlorine atoms obey the octet rule but the phosphorus atom does not. In case of SF4 also there are 10 electrons in the valence shell of sulphur.

Pocl3 Lewis Structure How To Draw The Lewis Structure For Pocl3 Youtube
Lewis Structures Lewis Structures Lewis Structures Are Representations

Chemistry Chemical Bonding 31 Of 35 Lewis Structures Exception To The Expanded Octet Rule Helpful Video When Get Octet Rule Secondary Science Chemistry

Lewis Structures Octet Rule Example Youtube

Resonance This Is The Lewis Structure We Would Draw For Ozone O3 Ppt Download
Chem 101 Octet Rule Violations

Lewis Structures Expanded Octet Pcl5 Janet Coonce Takes Time To Draw This Diagram Step By Step A Gre High School Science Secondary Science Teaching Science
Lewis Structures Octet Rule Example Youtube
3 Lewis Structure Octet Rule Lewis Dot Valence

Lewis Dot Structure Practice Problems With Answers And Explanation Youtube

No3 Lewis Structure How To Draw The Lewis Structure For No3 Chemistry Science Chemistry Chemistry Help

Enkelvoudige Ionen Samengestelde Ionen
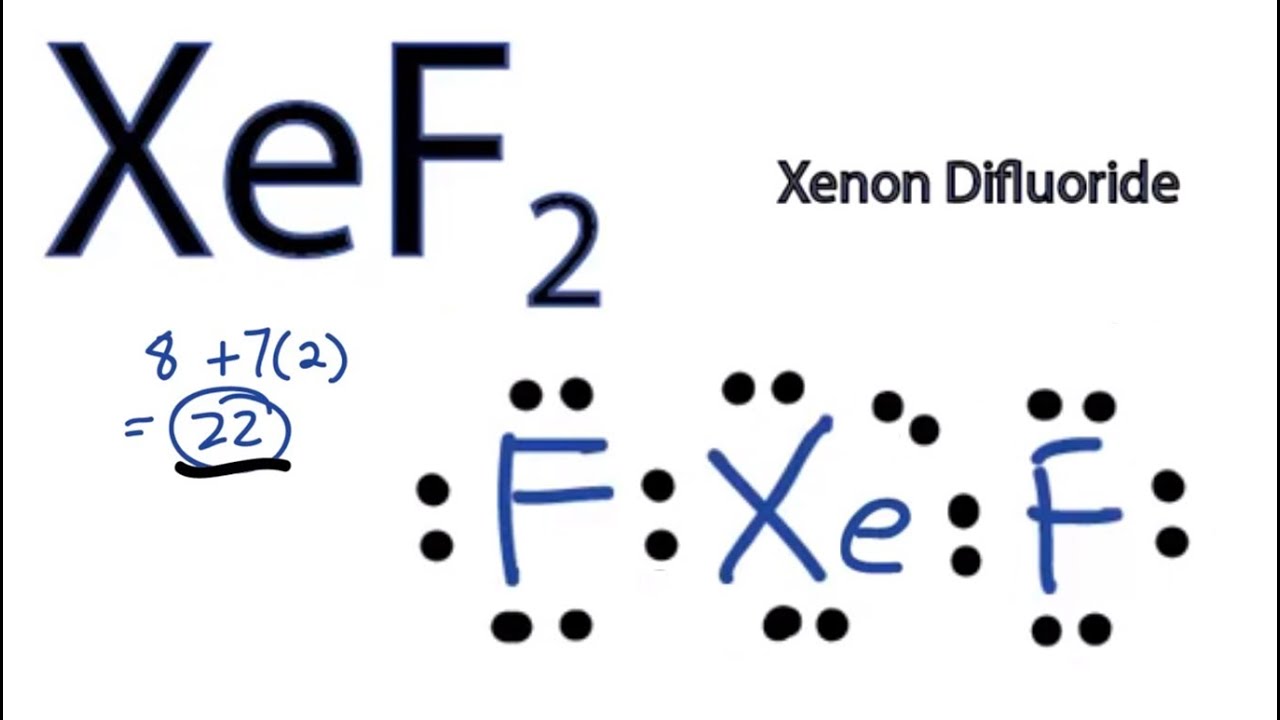
Xef2 Lewis Structure How To Draw The Lewis Structure For Xef2 Youtube

Formal Charges Calculating Formal Charge Practices Worksheets Chemistry Worksheets Secondary Science
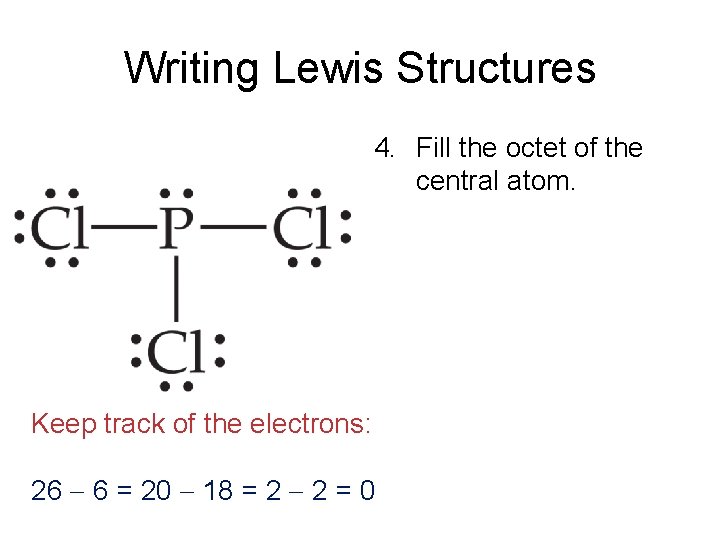
Lewis Structures Chapter 8 Lewis Structures Lewis Structures

Exceptions To The Octet Rule Lewis Dot Diagrams Youtube

Lewis Structures Chapter 8 Lewis Structures Lewis Structures

Socl2 Lewis Structure How To Draw The Lewis Structure For Socl2 Youtube
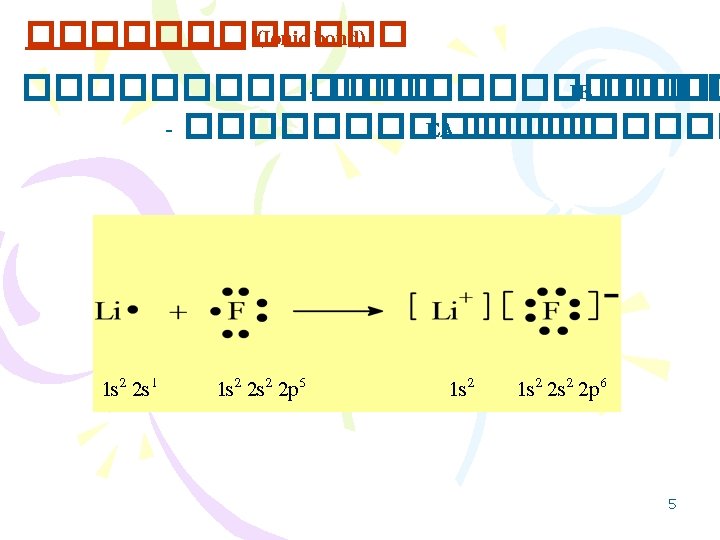
3 Lewis Structure Octet Rule Lewis Dot Valence