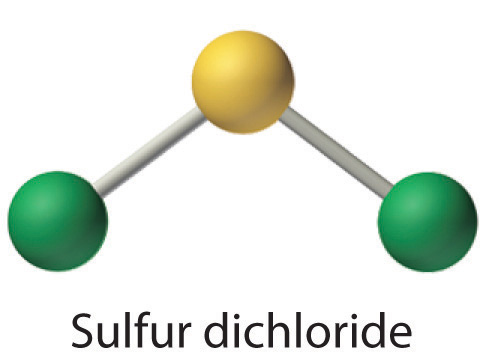
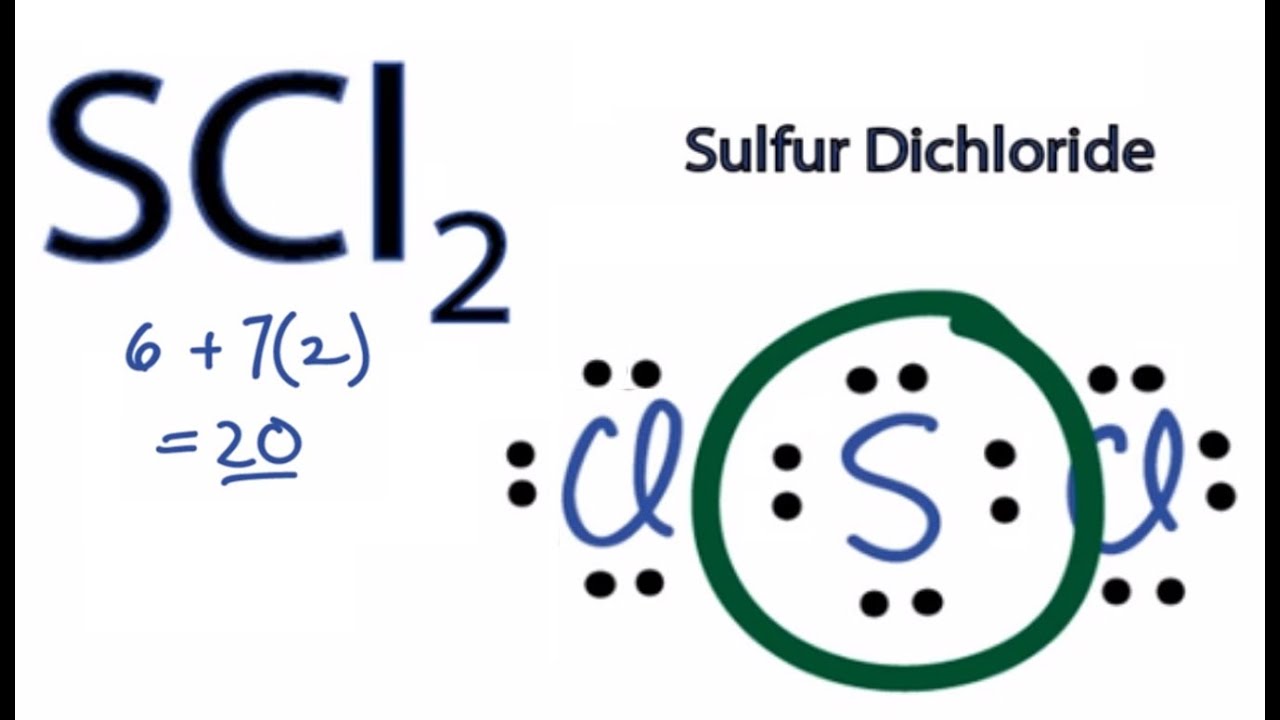
Scl2 Lewis Acid Or Base
Our tutors have indicated that to solve this problem you will need to apply the Lewis Acid and Base concept. However beryllium will then bear a negative charge so BeCl2 will only be a weak Lewis acid.
BASE wikipedia.

Scl2 lewis acid or base. Lewis acid is any species which accepts electron pair. This problem has been solved. In the Lewis theory of acid-base reactions bases donate.
The modern-day definition of a Lewis acid as given by IUPAC is a molecular entityand corresponding chemical speciesthat is an electron-pair acceptor and therefore able to react with a Lewis base to form a Lewis adduct. Hence they have electron surplus and should have lone pair of electrons. This product is also referred to as a Lewis adduct.
Provide an example of a reaction. While SOCl2 has a lone pair of electrons that might make one think it would be a Lewis base its electropositive center at the sulfur makes it an acid. The other problems of this type involved cations or anions so I was able to identify the cation as the Lewis acid and the anion as the.
Lewis base is any species which donates electron pair. CeSO2g H2Ol H2SO3aq What I tried. Lewis acids and bases are described by the Lewis theory of acid-base reactions as electron-pair acceptors and electron pair donors respectively.
Is SiCl4 a Lewis acid or base. The other way in which. This defines an acid as something that can accept electrons.
Carbon accepts a pair of electrons so CO 2 is the Lewis acid. Most compounds considered to be Lewis acids require an activation step prior to formation of the adduct with the Lewis base. In some cases the Lewis acid is capable of binding two Lewis base a famous example being the formation of hexafluorosilicate.
Is SiCl4 a Lewis acid or base. If you want to quickly find the word you want to search use Ctrl F then type the word you want to search. According to the Bronsted-Lowry and Lewis acid-base theory the definition of Lewis acid is any substance that can accept a lone pair of electrons.
A Cu 2 is because Cu 2 is electron deficient cation. The second theory is the Lewis Acid-Base Theory. BeCl2 Beryllium dichloride is lewis acid What is an acid base neutral.
Is SiCl4 a Lewis acid or base. A step-by-step explanation of how to draw the H2CO3 Lewis Dot Structure Carbonic AcidFor the H2CO3 structure use the periodic table to find the total numb. Since the central beryllium atom in BeCl2 has four electrons around it it still has room to accept more.
A Lewis acid is an electron pair acceptor. A Lewis base is an electron pair donor. Provide an example of a reaction.
In this reaction each chloride ion donates one lone pair to BeCl 2 which has only four electrons around Be. By this definition SOCl2 is not an acid. Through this definition substances like BF3 and SOCl2 are classified as Lewis Acids.
You can view video lessons to learn Lewis Acid and Base. Therefore a Lewis base can donate a pair of electrons to a Lewis acid to form a product containing a coordinate covalent bond. See the answer See the answer See the answer done loading.
Hence it is Lewis acid as it can accept lone pair of electrons. A Lewis acid can accept an electron pair. List molecules Acid and Base.
Ill tell you the Acid or Base list below. This is accomplished by sharing the electron pair furnished by the Lewis base. The tin atom in SnCl 4 can expand its valence shell by utilizing a.
One of the most commonly-encountered kinds of Lewis acid-base reactions occurs when electron-donating ligands form coordination complexes with transition-metal ions. Identify the Lewis acid and Lewis base in each of the following reactions. Thus the chloride ions are Lewis bases and BeCl 2 is the Lewis acid.
In the Lewis model the H ion is the active species it accepts a pair of electrons from the OH-ion to form a covalent bond. Hence they are electron deficient. An acid is a molecule or ion capable of donating a hydron proton or hydrogen ion H or alternatively capable of forming a covalent bond with an electron pair a Lewis acid.
BeCl2 is lewis Acid. Lewis Acids and Bases. SiF 4 2 F SiF 6 2 Complex Lewis acids.
Lewis suggested another way of looking at the reaction between H and OH-ions. Provide an example of a reaction. Classically the term Lewis acid was restricted to trigonal planar species with an empty p orbital.
Or if you need more Lewis Acid and Base practice you can also practice Lewis Acid and Base practice problems. In the Brnsted model the OH-ion is the active species in this reaction it accepts an H ion to form a covalent bond. The chloride ion contains four lone pairs.

Stories Science Education And Tutorials

Using The Octet Rule Write The Lewis Formulas For A Scl2 B Ccl4 C Nf3 And D Ch2c Ch3 2 Brainly Com

Scl2 Lewis Structure Sulfur Dichloride Youtube

Welcome To Chem Zipper Com 2020

Scl2 Lewis Structure Molecular Geometry Or Shape Polarity Hybridization

Scl2 Lewis Structure Molecular Geometry Or Shape Polarity Hybridization

Scl2 Lewis Structure Molecular Geometry Or Shape Polarity Hybridization
Sulfur Dichloride Cl2s Pubchem
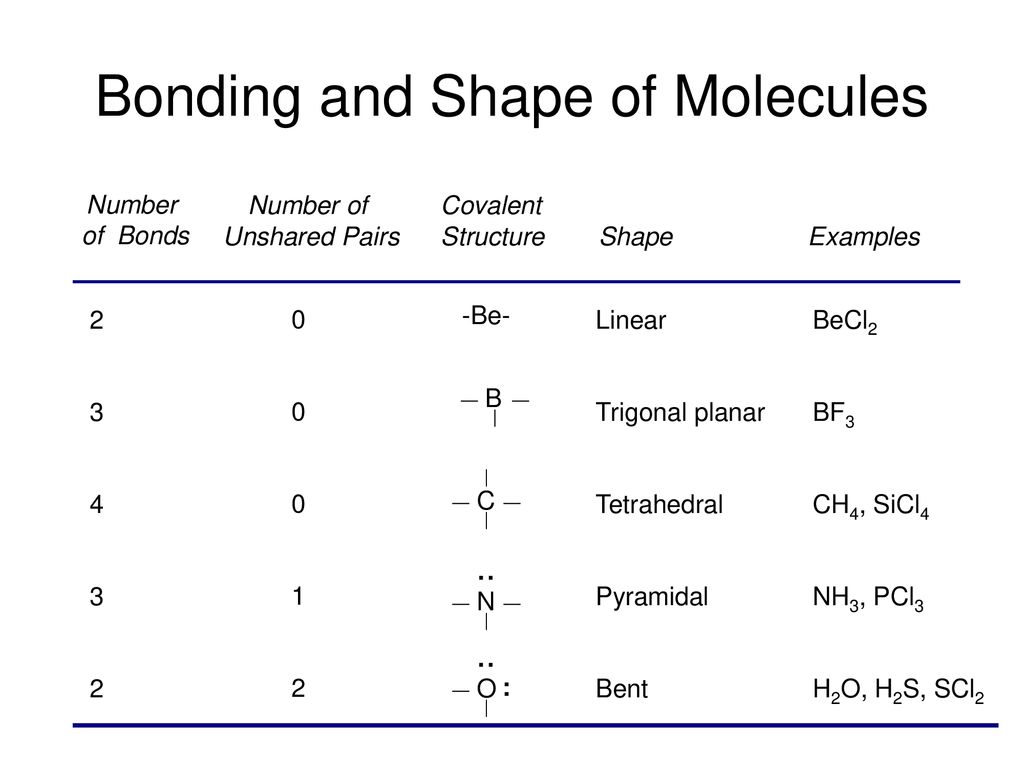
Molecular Models Activity Ppt Download

Scl2 Lewis Structure Sulfur Dichloride Youtube
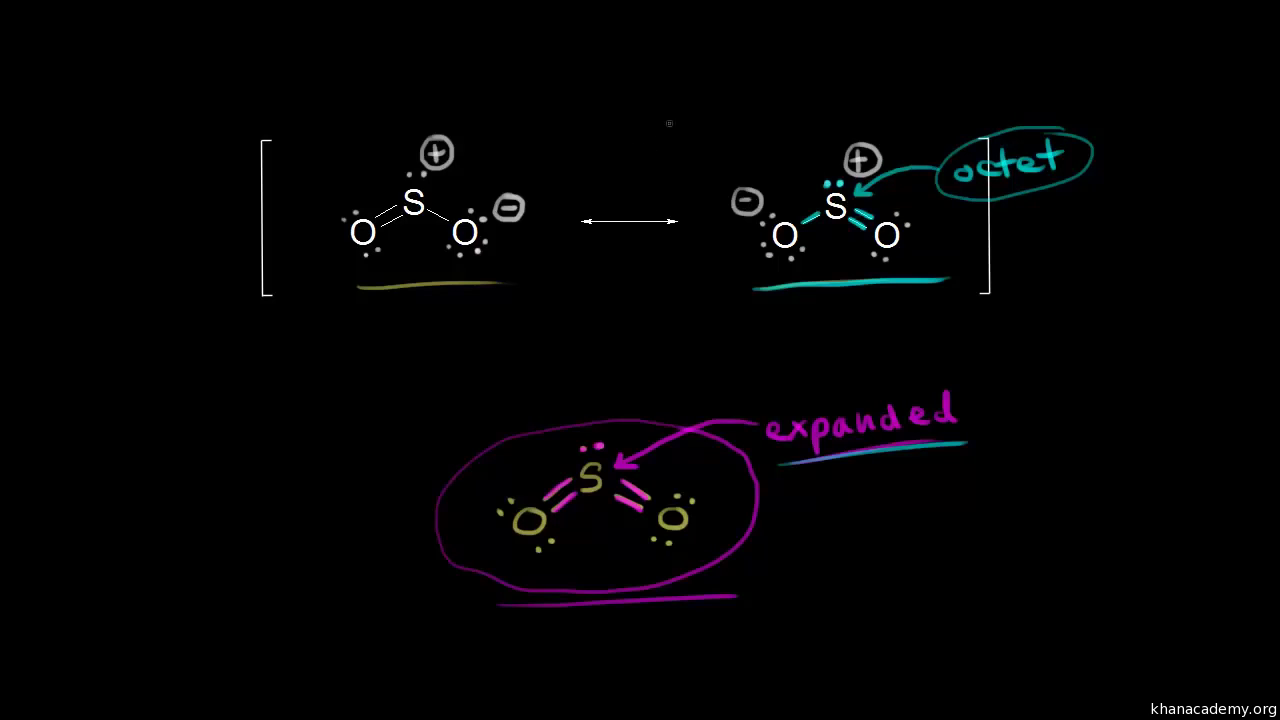
Sulfor Dioxide Lewis Dot Structure For So2 Video Khan Academy

Scl2 Lewis Structure Molecular Geometry Or Shape Polarity Hybridization

Scl2 Lewis Structure Sulfur Dichloride Youtube
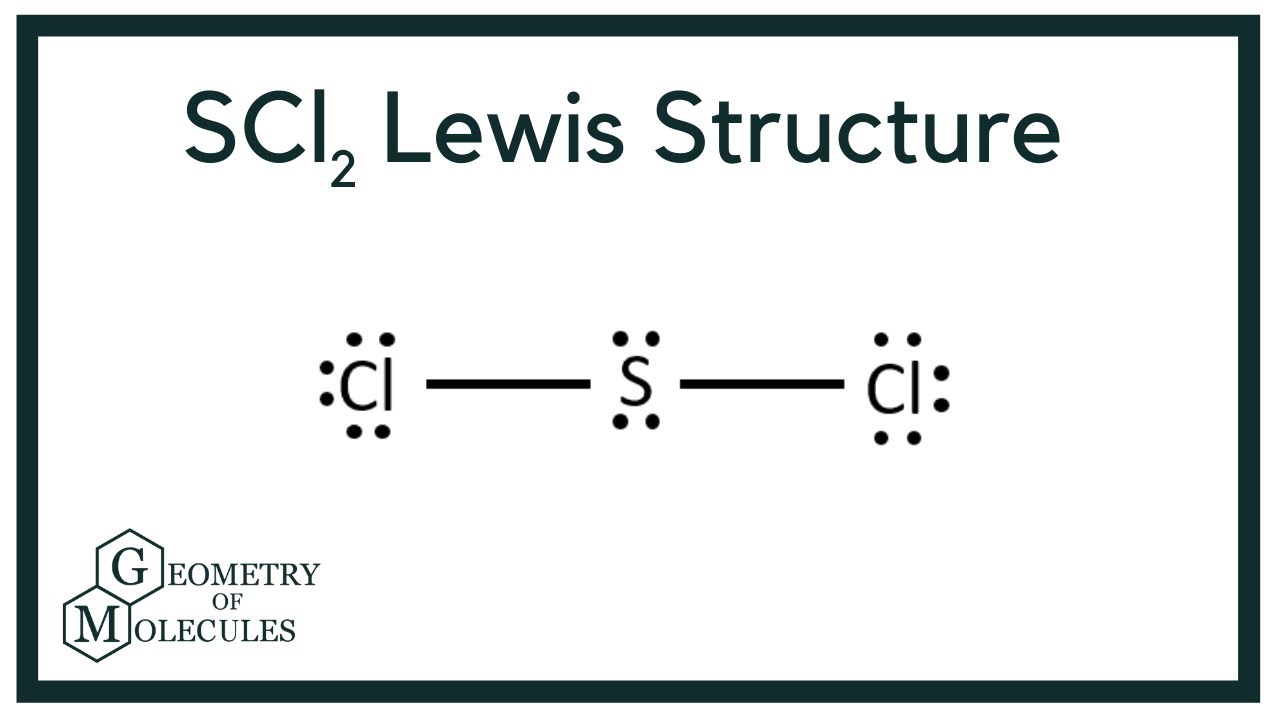
Scl2 Lewis Structure Sulfur Dichloride Youtube

H2s Molecular Geometry Science Education And Tutorials