Valence Electrons Of H3o+
6 Polar PNon-polar NP 7 Total number of unshared pairs of electrons. Start by forming chemical bonds.

What Is The Shape Of H3o Ion Quora
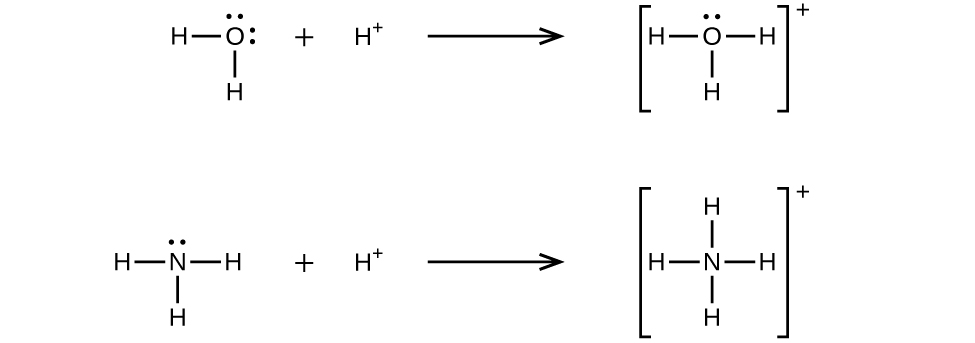
How does hydronium H3O form when the valence electron shell of water it already full of 8 electrons.

Valence electrons of h3o+. Oxygen has 6 valence electrons in its outer shell but as we know there are 4 oxygen atoms in this molecule. By looking at the group number you can tell how many valance electrons there are ex. Lewis dot structure of h3o hydronium ion.
HCl H2O H3O BCl3 NH3 NH4 CO2 CO IO2- NO3-SO42- BrO3- CH4 CH3Cl H2CO HCN. Therefore we only have 8 valence electrons for the h3o lewis structure. After that to complete the lewis structure of H3O we have to fill up the octet.
For the H3O Lewis structure we first count the valence electrons for. Valence electrons given by. To calculate the valence electrons in a molecule determine first the valence electron contribution of each atom.
So in keeping with the vsepr chart h3o has trigonal pyramid as its molecular form and tetrahedral as its electron geometry. I quickly take you through how to draw the lewis structure of hydronium ion h3o. Total number of valence electrons Valence electrons of Hydrogen Valence electrons of SulfurValence electrons of Hydrogen Sulfur has 6 valence electrons in its outer shell.
In this case we have oxygen atom as central atom so only oxygen will undergo hybridization. ANSWER 0 Science Whiz ANSWERS. The chemical formula Acetic Acid CH3COOH.
But we have 3 Hydrogens. 1 Total number of valence electrons. Is non-polar and explanation either using a diagram or in words involving no net dipole moment.
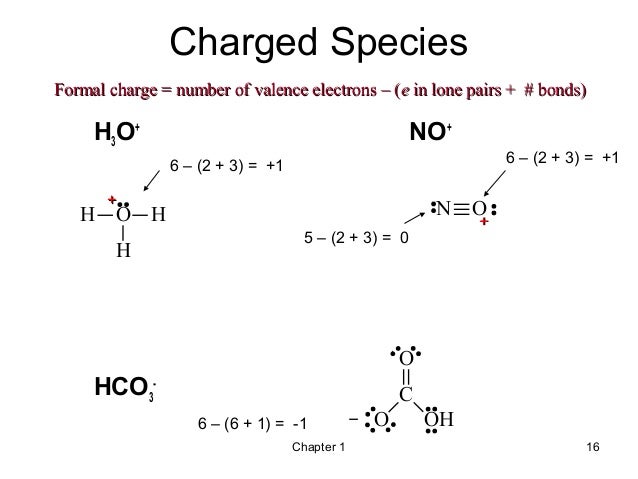
In this case Oxygen is the central atom. Also go over hybridization shape and bond angle. Note that the sign in the Lewis structure for H3O means that we have lost a valence electron.
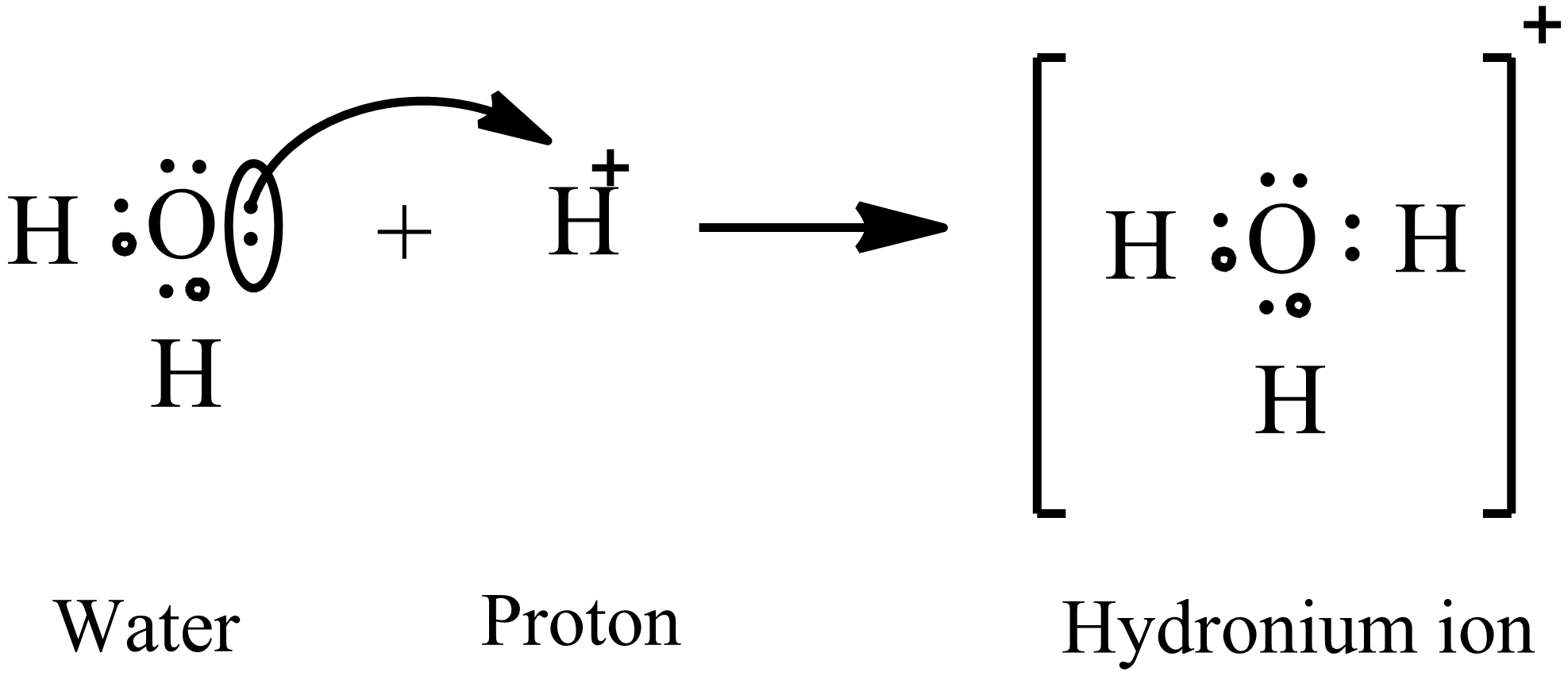
A step-by-step explanation of how to draw the H3O Lewis Structure Hydronium Ion. H doesnt have any electrons so you can stick them on anything that does have a a complete set of 8 and they will share 2 electrons of those 8. Water only has one such atom oxygen so thats where the H sits.
If this bond didnt break the hydrogen would have 4 electrons in its valence shell violating the filled shell ruleThe electron pair from the bond that breaks becomes a. For we first count valence electrons f. 3 Electron pair geometry.
Craig beals shows how to draw the lewis structure for hydronium ion. Lets do the Lewis structure for H3O the hydronium ion. Hence the valence for the acetic acid is 24.
Hence we will multiply the number by 4. Lets put the Oxygen at the center. Lets put some valence electrons around the atoms.
Next we wish to draw a skeletal construction of H3O with unmarried bonds best. Answer verified by Toppr. There are 8 valence electrons for the H3O Lewis structure.
Therefore we only have 8 valence electrons for the H3O Lewis structure. Next we need to draw a skeletal structure of H3O with single bonds only. Once it is on you cant.
Hydrogens always go on the outside right like that. Sign indicates gaining an electron Thus the total valence electron is 8 now. Each of the hydrogen atoms contributes 1 valence.
The valence of the carbon 4 hydrogen 1 and oxygen 6. As the new ceO-H bond begins to form the ceH-O bond in the hydronium ion begins to break arrow 2. That gives us 3 6 is 9 minus 1.
Charge of valence electrons nonbonding val bonding el. Lets put some valence electrons around the atoms. The electrons that participate in bond formation are.
On the periodic table Hydrogens in group 1 1 valence electron. Thus the total valence electron is 8 now. H3O is an important compound in.
Covalent bonding 23 structures with. Secondly we need to resolve a central atom which is typically the atom with probably the most available sites for bonding. H3O Trigonal pyramidal.
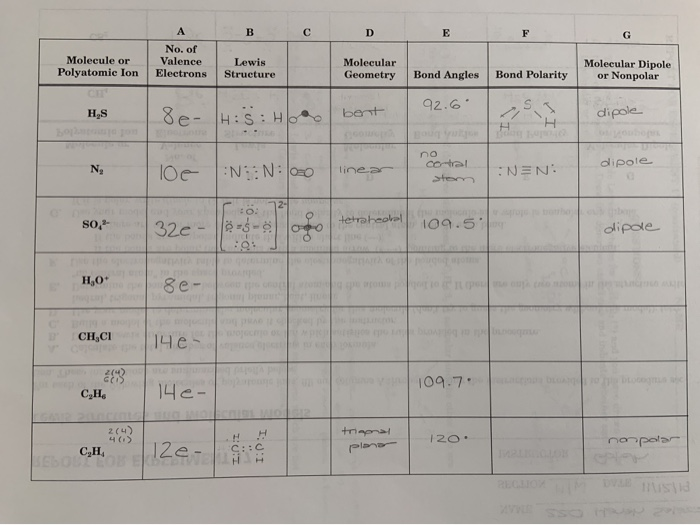
Group 1 1 valance electrons Group 2-12 2 valance electrons Group 13 3 valance electrons. 109 s polar and explanation either using a diagram or in words involving the net dipole moment C2H4 Trigonal planar. To find out total valence electrons given by a particular element you should multiply number of electrons of the valance shell by the number of atoms of that element.
In this case Oxygen is the central atom. Secondly we need to determine a central atom which is generally the atom with the most available sites for bonding.
Please Help Me With H3o Ch3cl C2h6 And C2h4 The Chegg Com

A Step By Step Explanation Of How To Draw The H3o Lewis Structure Youtube

H3o Lewis Structure Geometry Hybridization And Mo Diagram Techiescientist

A Step By Step Explanation Of How To Draw The H3o Lewis Structure Youtube
What Is The Bond Angel Of H3o Quora

Electron Dot Structure Of Hydronium Ion Brainly In

126 Hso4 Lewis Structure How To Draw The Lewis Structure For The Bisulfate Ion Youtube Science Chemistry Chemistry Organic Chemistry
15 2 Lewis Acids And Bases Chemistry

How Can H3o Be Formed Thought The Valence Electrons Of Oxygen Are 2 Quora
Draw An Electron Dot Diagram To Show The Structure Class 12 Chemistry Cbse

H3o Lewis Structure Geometry Hybridization And Mo Diagram Techiescientist
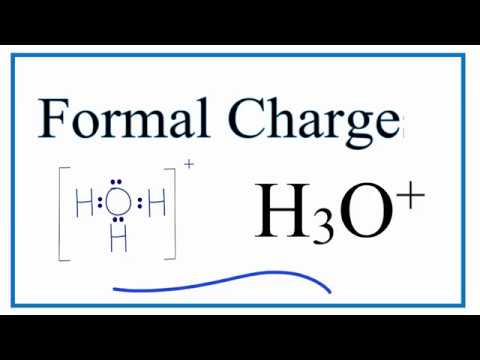
How To Calculate The Formal Charges For H3o Hydronium Ion Youtube
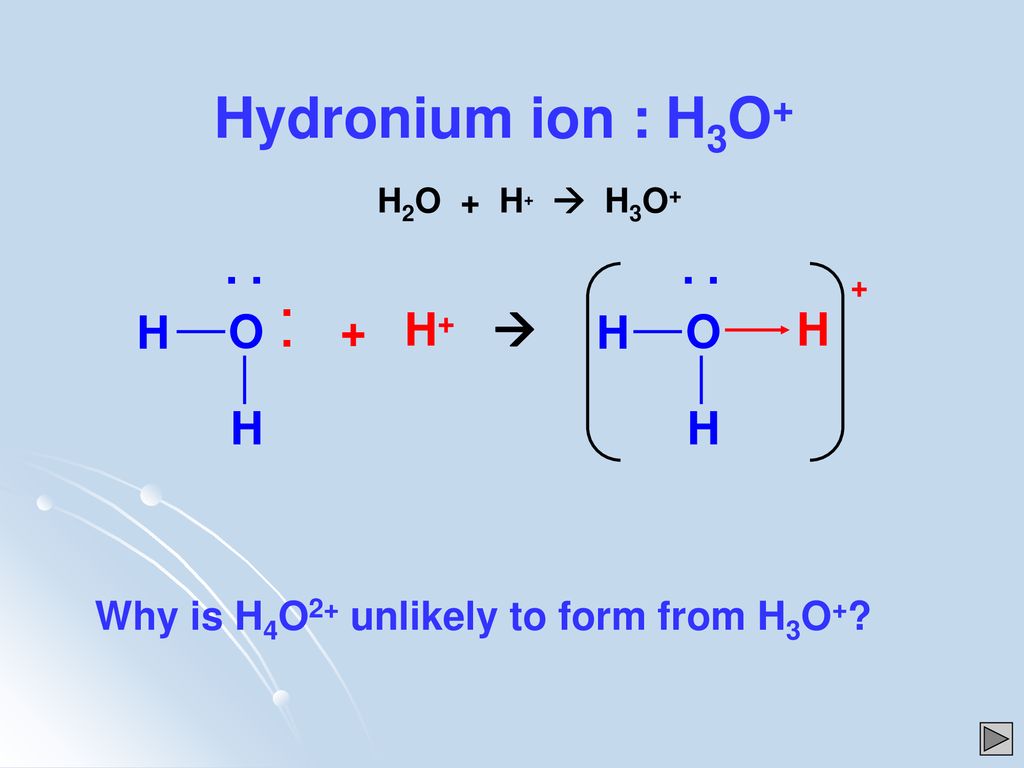
As Chemistry Coordinate Or Dative Covalent Bonding Nh4 Al2cl6 Co H3o Ppt Download

H3o Lewis Structure Geometry Hybridization And Mo Diagram Techiescientist
What Is The Shape Of H3o Ion Quora

Chemistry Chemical Bonding 22 Of 35 Lewis Structures For Ions Hydronium Ion H3o Youtube
Electron Dot Structure Of Hydronium Ion