What Is The Lewis Structure Of C2h4
Drawing the Lewis dot structure for C2H4 ethene and answer the questions below. This is composed of a σ framework and a π-bond.
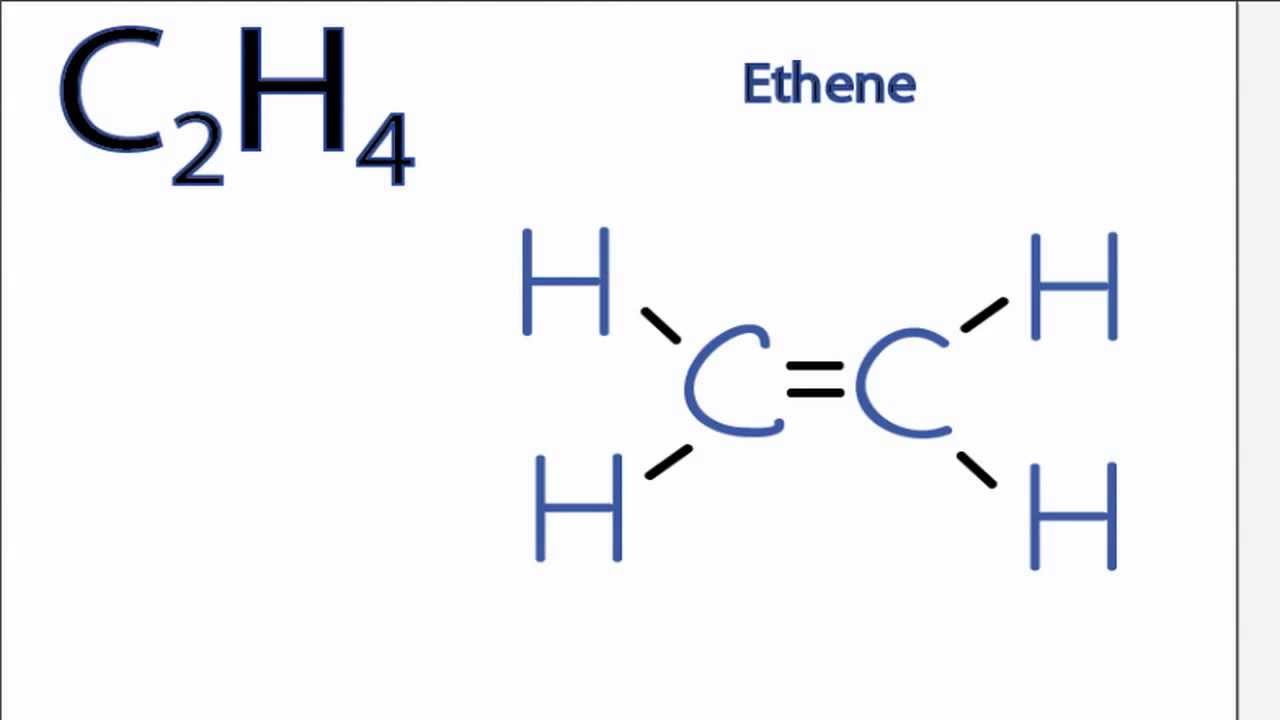
Is C2h4 Polar Or Nonpolar Youtube
Count total valence electron in C2H4.
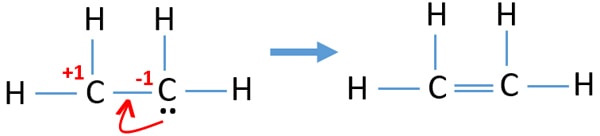
What is the lewis structure of c2h4. We place two valence electrons between each atom as shown in the figure. Look at the structure for each molecule and determine if your lewis structure and shapes were correct. Lewis Dot Structure for C2H4 6 of 6 Watch the video of Dr.
Electron dot structure of C2H4 H2CCH2. It is referred as gas olefiant or oil making gas. 1 point for the correct selections assessed when you answer and 5 points for the Lewis structure on your work assessed when I review.
For C 2 H 4 you have a total of 12 total valence electrons. The molecular shape is predicted to be trigonal planar around each carbon atom. In a double bond two pairs of valence electrons are shared for a total of four valence electrons.
There are two triangles overlapping each other as we can see in. Draw the Lewis structure for C2H4. B What is the hybridization of the carbon atoms in each.
For C2H4 you have a total of 12 total valence electrons. Welcome to Geometry of Molecules and today in this video we are going to help you know the step-by-step method for determining the Lewis Structure. According to the VSEPR chart the shape of the ethene molecule is trigonal planar.
Use information from step 4 and 5 to draw the lewis structure. Note that the C 2 H 4 Lewis dot structure involves sharing more than one pair of electrons. Drawing the Lewis Structure for C 2 H 2 Ethyne or Acetylene.
Is c2h4 polar or nonpolar simple is that c2h4 is. Alternatively a dot method can be used to draw the lewis structure. This means that the carbon atoms share 4.
What is the shape of C2H4. Drawing the Lewis structure for C2H4 named ethene requires the use of a double bond. Calculate the total valence electrons in the molecule.
H-----C---H SHOW WORK This question is worth a total of 6 points. C2H4 Lewis Structure. The Lewis structure of C 2 H 4 also known as ethene has two carbons with a double bond between them.
Ethylene C2H4 is the simplest alkene and because it contains a double bond it an unsaturated hydrocarbon. This structure helps in understanding the arrangement of valence electrons around the atoms in the molecule. In C2H4 if we look into the lewis structure we will see that there are three bonded pairs of electrons around each carbon and zero lone pair.
Hydrogen is the least electronegative element here. Drawing the Lewis structure for C 2 H 4 named ethene requires the use of a double bond. Lewis structure of C2H4.
C2H4 Lewis structure contains four C-H bonds and one double bond in between two carbon atoms. Arrangement of atoms shown below dashed lines show connections between atoms. Remember that hydrogen atoms always go on the outside of a Lewis structure and that they only need two valence.
Submit Your â Here a 25 Å resolution structure of four ZF domains of Snail1 complexed with importin β is presented. A step-by-step explanation of how to draw the C2H2 Lewis Dot Structure Ethyne or AcetyleneFor the C2H2 structure use the periodic table to find the total. There are only single bond between carbon atom and hydrogen atom because hydrogen caannot keep more than two electrons in its last shell.
This means that the carbon atoms share 4. However in Hydrocarbons we always place the Carbon atoms in the center as shown in the figure. Draw the Lewis structures of C2H6 C2H4 and C2H2Draw the molecules by placing atoms on the grid and connecting them with bonds.
What is the electron dot formula of C2H4. To draw the c2h4 lewis. In the lewis structure of C 2 H 4 there are only four C-H bonds one CC bond and no lone pairs on last shells.
In a double bond two pairs of valence electrons are shared for a total of four valence electrons. Lewis dot structure of C 2 H 4. No lone pair is present on the central or outer atom in the lewis structure of ethene.
What is the Lewis structure of C2H2. The lewis structure of C2H4 is very easy to draw-Some steps need to follow for drawing the C2H4 Lewis dot structure 1. We have 12 available valence electrons.
Its chemical formula is C2H4 where there is a double bond between the carbons. Drawing the Lewis Structure for C 2 H 4. The formula for this is C2H4It has four hydrogen atoms bound.
C2H4 has the Lewis Structure. As Carbon is the least electronegative atom in this molecule it will take the central position. Start by forming covalent bonds between the Carbon and Hydrogen atoms.

Ethene C2h4 Lewis Structure Hybridization

Draw The Electron Dot Structure Of Ethene C2h4 Brainly In
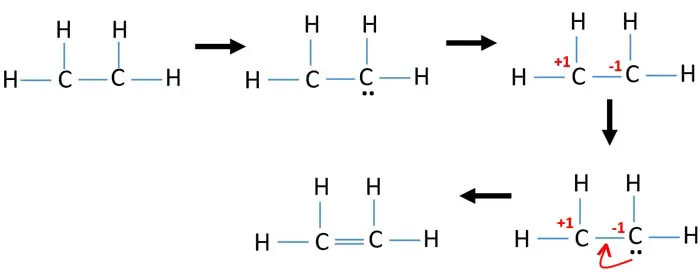
Ethene C2h4 Lewis Structure Hybridization

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

C2h4 Lewis Structure C2h4 Lewis Structure Molecular Geometry

Draw The Lewis Structure For Ethylene C2h Clutch Prep
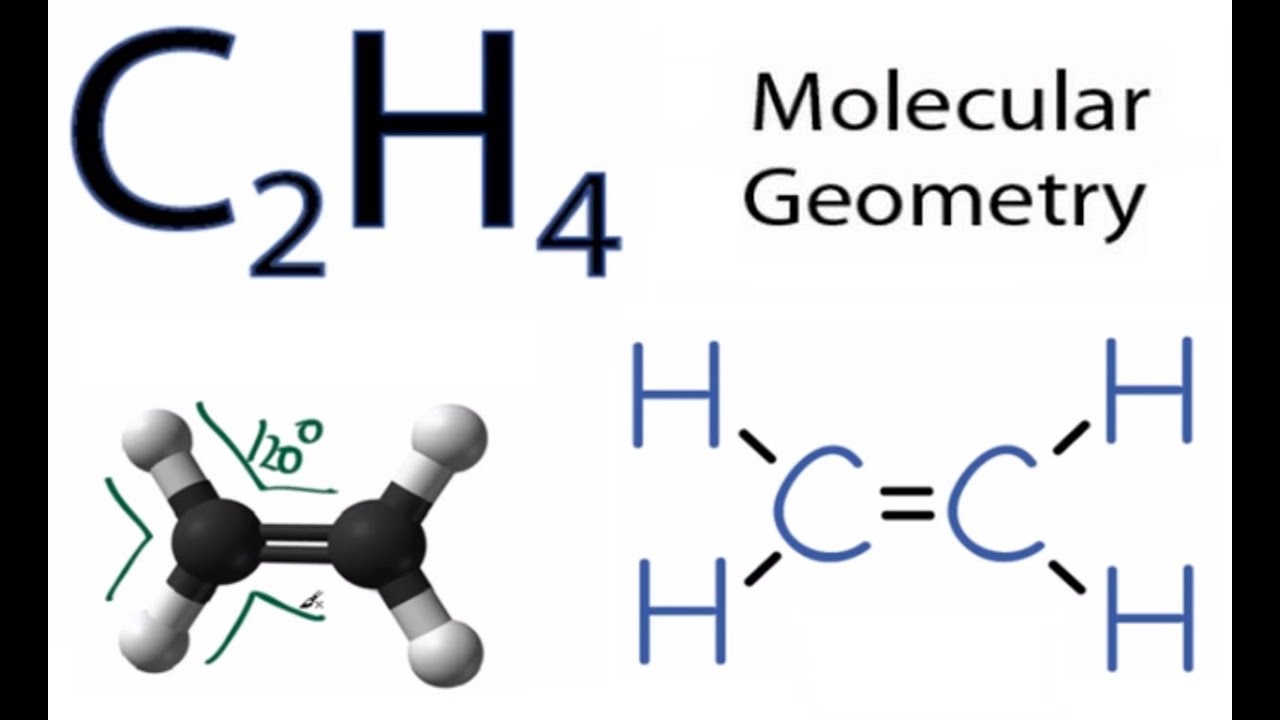
C2h4 Molecular Geometry Shape And Bond Angles Youtube
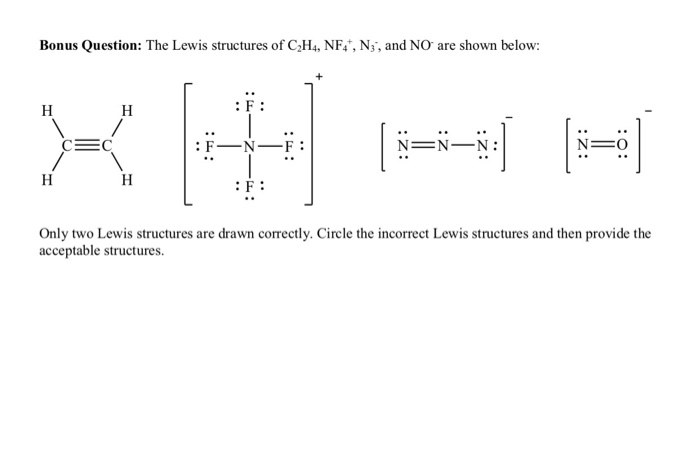
Bonus Question The Lewis Structures Of C2h4 Nf N Chegg Com

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

Draw The Lewis Structure For The C2h4 Ske Clutch Prep

Draw The Lewis Structure For Ethylene C2h4 Be Certain You Clutch Prep
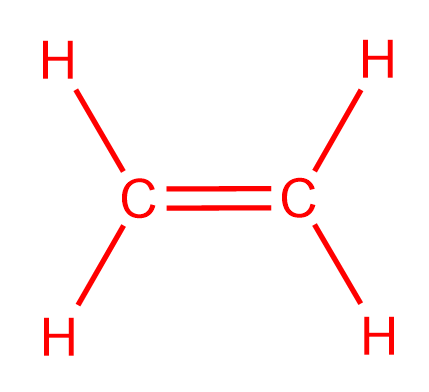
Draw The Lewis Structure For The C2h4 Ske Clutch Prep

Write Lewis Structure Of C2h4 Brainly In

C2h4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
![]()
Draw And Explain The Lewis Structure Of C2h4 Study Com
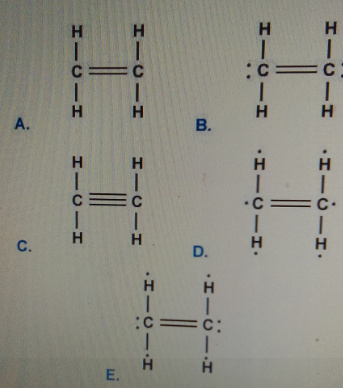
Which Is The Correct Lewis Structure For Ethylene C2h4 Home Work Help Learn Cbse Forum

Two Important Industrial Chemicals Ethene C2h4 And Propene C3h6 Are Produced By The Steam Or Thermal Cracking Process 2c3h8 G Rightarrow C2h4 G C3h6 6 Ch4 G H2 G For Each Of The Four
Lewis Structure Of C2h4 Biochemhelp

C2h4 Lewis Structure Molecular Or Electron Geometry Polar Or Nonpolar