What Is The Molecular Shape Of Ncl3
NCl3 is a slightly polar molecule because of the small difference between the electronegativity of nitrogen and chlorine atom. You now have to locate the two lone pairs.
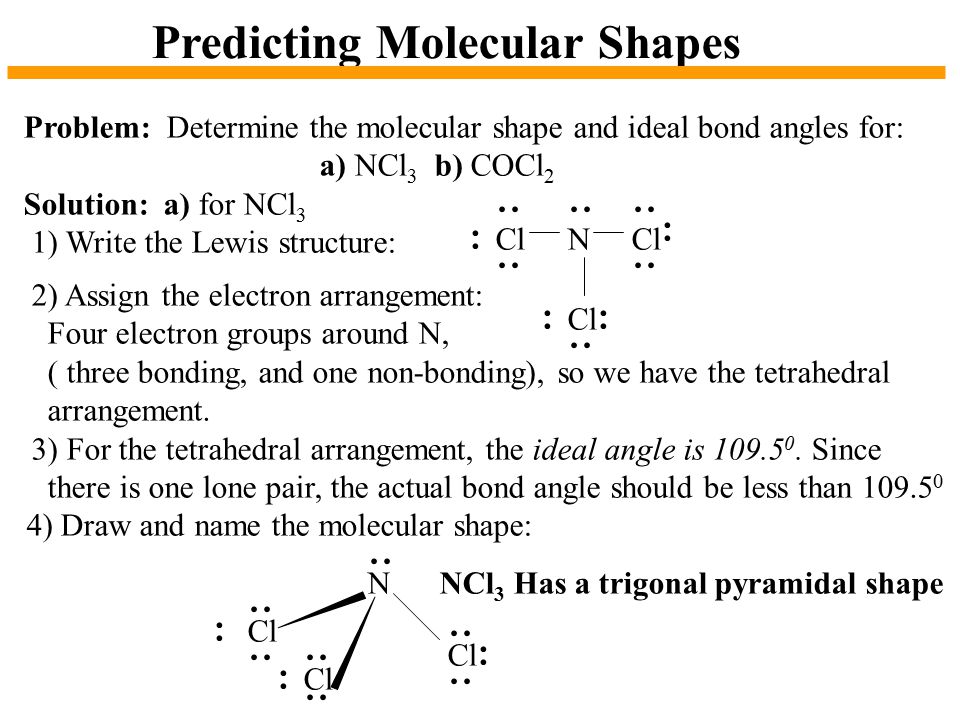
Chapter 10 The Shapes Of Molecules Ppt Video Online Download
These five electron pairs arrange themselves into a trigonal bipyramidal orientation.

What is the molecular shape of ncl3. NCl3 molecular geometry consists of a trigonal pyramid structure. Sign up for free to see the solution. If so feel free to rate me ath.
A linear b trigonal planar c bent d tetrahedron e trigonal pyramid. Our mission is to help you succeed in your Chemistry class. The molecular structure of NCl3 is a trigonal pyramidal b none of these c octahedral d trigonal planar e bent.
There are one Lewis structure of NCl3can be drawn by using valence electrons of nitrogen and chlorine atoms. The Correct Answer is. See full answer below.
Iodine starts off with 7 electrons and each chlorine provides it with 1 giving a total of 5 pairs. What is the Lewis structure of nf3. NCl3 molecule has one lone pair that leads to repulsion between electrons and the shape of the molecule is trional pyramidal.
The N-Cl distances are 176 Å and the Cl-N-Cl angles are 107. Sign up to view answer. Its electron shape would be tetrahedral that is when you count the lone pairs of electrons as bonds themselves.
An NCl3 molecule would be a trigonal pyramidal because it has one center N atom with 3 Cl surrounding it but also a lone pair of electrons on the top which bends the molecule downward forming a trigonal pyramidal. Like ammonia NCl 3 is a pyramidal molecule. Learn how to draw a Lewis Structure and how to use it to assign a molecules shape geometryAre you taking a class with me.
Chemistry questions and answers. NCl3 takes this shape due to it following the AX3 E1 format. NF3 nitrogen trifluoride is very similar in structure to NCl3 and NH3 Lewis.
Use VSEPR theory to predict the molecular geometry of nitrogen trichloride NCl3. It is polar because its charges are distributed asymmetrically and its geometric shape is asymmetricalPolar Molecules. Thus the net dipole moment is 0.
Nitrogen trichloride is a yellow oily liquid with its pungent odor. Nitrogen N is the least electronegative element and is at the center of the Lewis structure of NF3. According to the VSEPR theory the molecular geometry of NCl3 is trigonal pyramidal and electron geometry is tetrahedral because nitrogen being pentavalent has Sp³ hybridization with 5 valence electrons in its outermost shell and it makes three bond pairs one with each chlorine atom.
2 or more lines of symmetry when you cut What is the molecular polarity of NCl3 and why. Hydrogen escapes into the Lewis NF3 structure a. In that way they will be at 120º to each other.
NCl3Nitrogen trichloride Lewis Structure There are 3 chlorine atoms and one nitrogen atom in NCl3. It has a molecular geometry of trigonal pyramidal which also looks like a distorted tetrahedral. The electron geometry of this molecule will be tetrahedral and it will have eqrmsrmprm3 eq hybridization.
These will both go into equatorial positions to minimise repulsions. NCl3lewis structure contains three N-Cl bonds. This means that the.
Is The Molecular Shape Of The NCl3 Molecule Straight Line linear Pyramidal Bent Trigonal Planar Or Tetrahedral. This is because the two equal bond dipoles point in opposite directions and cancel the effect of each other. The molecular geometry of the molecule will be trigonal pyramidal.
However BeF2 is a non-polar molecule and its dipole moment is zero. Is The Molecular Shape Of The NCl3 Molecule Straight Line linear Pyramidal Bent Trigonal. The hybridization in BeF2 is sp thus it is linear in shape.
7 rows NCl3 from the air environment reacts with DPD 3 releasing iodine which reacts with DPD 1 and. Is The Molecular Shape Of The NCl3 Molecule Str. The Lewis structure NF3 has a total of 26 valence electrons.

The Molecular Structure Of Ncl3 Is A Trigonal Pyramidal B Clutch Prep
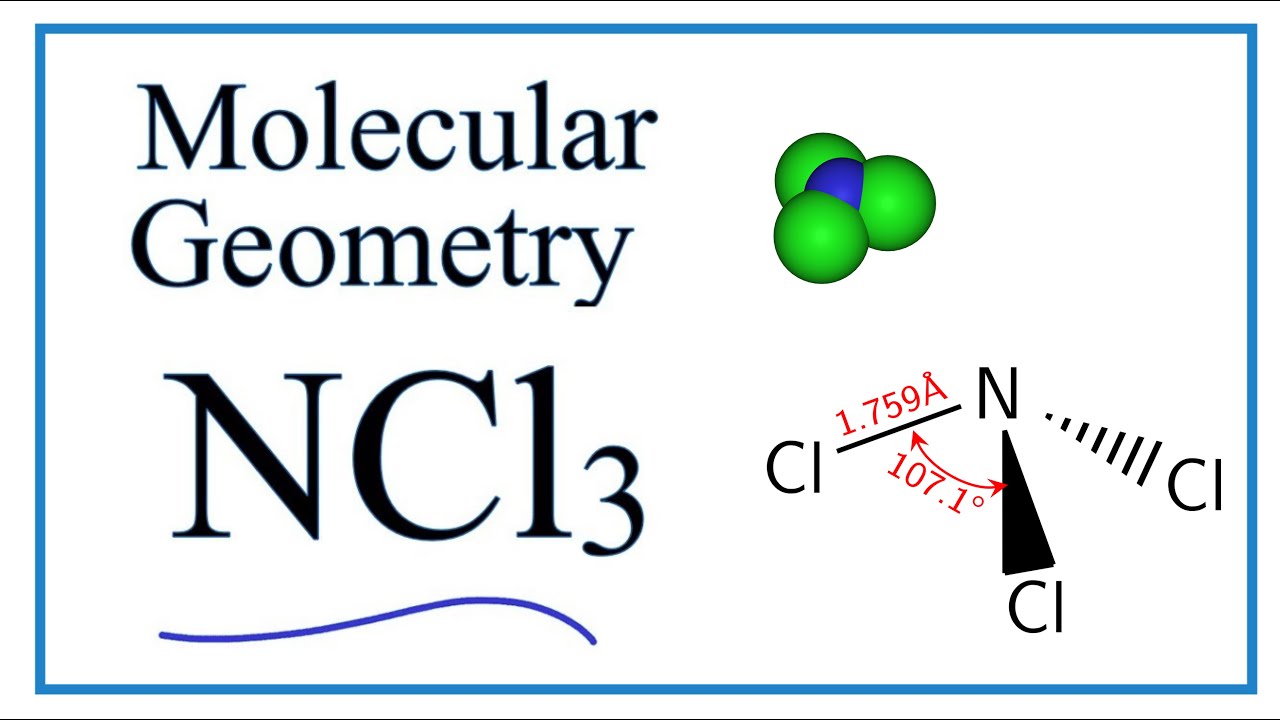
Ncl3 Molecular Geometry Shape And Bond Angles Youtube

Determine The Electron Geometry Eg And M Clutch Prep
What Is The Molecular Geometry Of Ncl3 Quora

Ncl3 Lewis Structure How To Draw The Dot Structure For Ncl3 Youtube

Is Ncl3 Polar Or Nonpolar Techiescientist
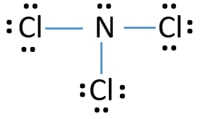
Ncl3 Nitrogen Trichloride Lewis Structure

The Total Number Of Lone Pairs In Ncl3 Is Study Com
Ncl3 Molecule Of The Month April 2017 Html Only Version

Choose A Lewis Structure For Ncl3 Clutch Prep

Choose A Lewis Structure For Ncl3 Clutch Prep

Ncl3 Lewis Structure Molecular Geometry Polar Or Nonpolar Bond Angle

Determine The Electron Geometry Eg And M Clutch Prep

Draw The Lewis Structure For Ncl3 And Provide The Following Information A Number Of Electron Groups B Electron Pair Geometry C Bond Angle D Number Of Bonded Electrons E Molecular Geometry F

Ncl3 Lewis Structure And Molecular Geometry Geometry Youtube

The Molecular Structure Of Ncl3 Is A Trigonal Pyramidal B Clutch Prep
Ncl3 Lewis Structure And Molecular Geometry Geometry Youtube
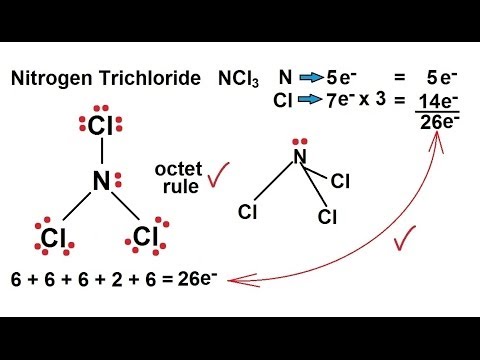
Chemistry Chemical Bonding 14 Of 35 Lewis Structures Nitrogen Trichloride Ncl3 Youtube

Draw The Lewis Dot Structure For Ncl3 Clutch Prep