Write The Lewis Structure For Co2
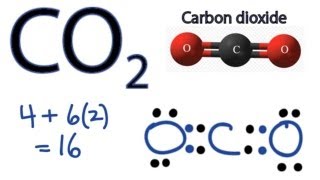
CO2 4 62 16. OCo But what exactly does this mean.
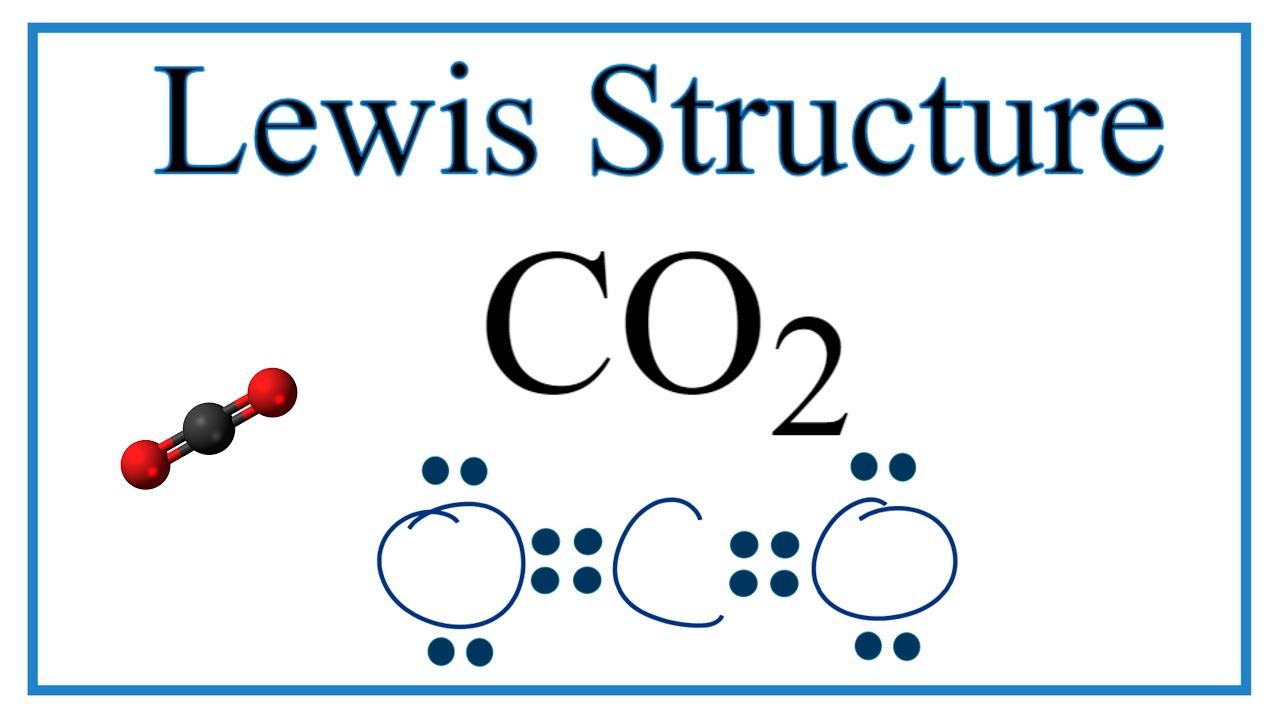
Lewis Dot Symbols And Lewis Structures Boundless Chemistry
Carbon is the least electronegative that means it is going to go to the center.

Write the lewis structure for co2. Write The Lewis Symbol For Each Atom Or Ion. Here are the steps that I follow when drawing a Lewis structure. Co2 lewis structure polar or nonpolar.
United Kingdom 48 7161 Orders Completed. You can always ask and chat with this writer about your homework needs. Lewis structure for CO2.
Lets draw the structure. Follow to get the latest 2021 recipes articles and more. The CO2 molecule has a total of 16 valence electrons 4 from carbon and 6.
Draw the molecule by placing atoms on the grid and connecting them with bonds. In Lewis Dot structure for CO2the carbon atom follows the. Draw the molecule by placing atoms on the grid and connecting them with bonds.
It is sometimes possible to write more than one Lewis structure for a substance that does not violate the octet rule as we saw for CH 2 O but not every Lewis structure may be equally reasonable. One of these oxygen atom take a proton H ion and form a. I Draw Lewis structure of.
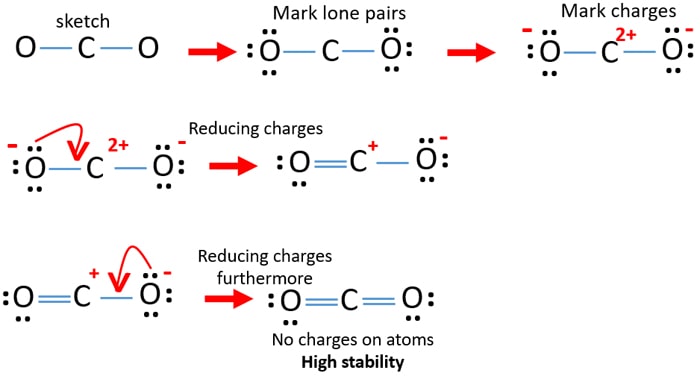
In carbonate ion there is two oxygen atoms which has -1 charge on each of them. Decide which is the central atom in the structure. Count the valence electrons in your trial structure.
I quickly take you through how to draw the Lewis Structure of CO2 Carbon DiOxide. Include all lone pairs of electrons and formal charges on atoms. Carbon dioxide or CO2 has three resonance structures out of which one is a major contributor.
From the periodic table Carbon has four valence electrons. The CO2 molecule has a total of 16 valence electrons 4 from carbon and 6 from each oxygen atom. That will normally be the least electronegative atom C.
Ii Why H2SO4 has an exception Lewis structure. You follow a sequence of steps. Put the Carbon at the center and then Oxygen on either side of that.
Write The Lewis Structure For H2co. A step-by-step explanation of how to draw the CO2 Lewis Dot Structure Carbon dioxideFor the CO2 structure use the periodic table to find the total number. Ii Why H2SO4 has an exception Lewis structure.
These cupcakes are my kids favorite snack to bring in their lunch boxes. Homework solution attached Purchase this answer to view it This homework is solved by this writer. They are so good that even the teachers would write me a.
I also go over hybridization shape and bond angles. Oxygen has six valence electrons. In order to complete the octets for all of the atoms in the structure you will need to form two double bonds.
Draw a skeleton structure in which the other atoms are single-bonded to the central atom. Write the lewis dot structure for i ii 11k. A CO32 b NH4 ions.
For Lewis structure of CO2 you will now have two Oxygen atoms forming double bonds with a Carbon atom. As all the valence electrons of all the atoms are used there are no lone pairs of electrons or non-bonding pairs of electrons in the molecule. Video about Write The Lewis Structure For Co2.
Classify the bond in CO2 as polar covalent non polar covalent or ionic. By darylfarahi Posted on June 7 2020day. This browser does not support the video element.
The Lewis Dot Structure for carbon dioxide can be represented like this. Lewis Dot structure for CO2 In Lewis Dot structure for CO2there are four lone pairsEach oxygen atom has two lone pairs around itThe carbon atom doesnt have any lone pair. Tasty Cheese Cup Cake.
Draw the Lewis structure for CO23. Draw the Lewis dot structure of CO2 molecule. Include all lone pairs of electrons and formal charges on atoms.
In these situations we can choose the most stable Lewis structure by considering the formal charge on the atoms which is the difference between the number of valence electrons in the free atom and the number assigned to it in the Lewis electron structure. Draw the Lewis structure for CO23. Carbon C is the least electronegative atom in the CO2 Lewis structure and therefore should be placed at the center of the structureThe Lewis structure for CO2 has a total of 16 valence electrons.
Asked Nov 27 2020 in Chemistry by Panna01 472k points. What is a Lewis Dot Structure and what do the symbols in carbon dioxides structur. Draw a trial structure by putting electron pairs around every atom until each gets an octet.
Carbonate lewis structure and bicarbonate lewis structure.

Co2 Lewis Structure Carbon Dioxide Youtube
How To Draw The Lewis Dot Structure For Co2 Carbon Dioxide Youtube

How To Draw The Lewis Structure Of Co3 2 Carbonate Ion Chemistry Youtube
Co2 Lewis Structure Molecular Geometry And Hybridization
![]()
A Draw Lewis Structures For Co2 So2 And No3 B Give The Electron Pair Geometry And The Molecular Geometry Of The Three Species From Part A According To Vsepr C Are Co2

Co2 Lewis Structure How To Draw Or Write The Lewis Dot Structure For Carbon Dioxide Youtube

Write Lewis Dot Structure Of Bf3 And Co2 Scholr

Co2 Lewis Structure How To Draw The Dot Structure For Carbon Dioxide Youtube

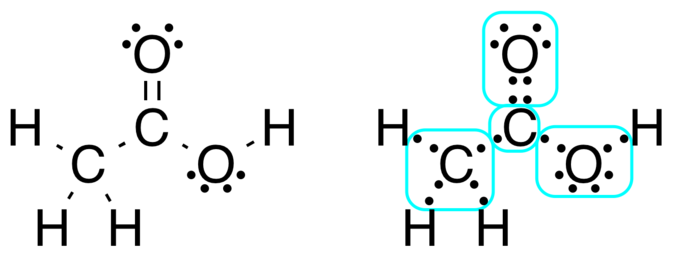
How To Draw The Lewis Dot Structure For Ch4 Methane Youtube

What Is The Lewis Structure Of Co2 Clutch Prep
Co2 Lewis Structure How To Draw The Dot Structure For Carbon Dioxide Youtube

Lewis Structure Practice Worksheet 4 Stepsa Ch4 Lewis Structure In 2020 Practices Worksheets Graphing Linear Equations Chemistry Worksheets
Co2 Carbon Dioxide Lewis Structure And Shape

Co2 Lewis Structure Easy Hard Science

Co2 Lewis Structure How To Draw The Dot Structure For Carbon Dioxide Youtube

Co32 Lewis Structure How To Draw The Lewis Structure For Co3 2 Carbonate Ion Youtube

Co2 Lewis Structure Easy Hard Science

Carbon Dioxide Lewis Structure How To Draw The Lewis Structure For Carbon Dioxide Youtube

Draw The Lewis Dot Structure Of Co2 Molecule