Write The Lewis Structure For Pcl3
You can also find the potential energy function of H-Cl and take the derivative with respect to internuclear distance and find minimum. Write Lewis structures for the following.
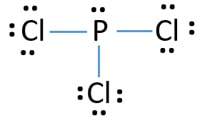
Pcl3 Phosphorus Trichloride Lewis Structure
Write the Lewis structure for the diatomic molecule P 2 an unstable form of phosphorus found in high-temperature phosphorus vapor.
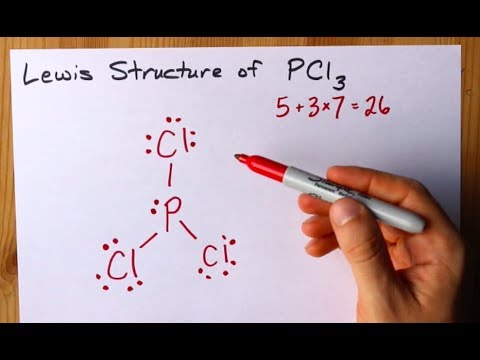
Write the lewis structure for pcl3. Write Lewis Structures For. How many pi bonds in the formula. Draw the lewis structure of PCl 3.
Draw the Lewis structures of magnesium and oxygen by drawing two dots around Mg and six dots around O 2. Write all of the Lewis symbols on the same sheet of paper and upload the file. So little structure H.
Drawing Lewis Structures A Tutorial on Writing Lewis. You know what for D s F two to impairs on sulfur. Answer all questions related to the drawing.
If you are interested in this advanced topic see. H 2 CCH 2. How many sigma bonds in the formula.
Draw the Lewis structure for hydrogen the simplest molecule and explain the drawing. What is the Lewis dot structure for PCL3. Also there is a lone pair on phosphorus atom.
Drawing the Lewis Structure for PCl 5. PCl 3 phosphorus is in the middle with one lone pair and is surrounded by single bonds to the three chlorines each with 3 lone pairs. B GeF4 c NO2 e BBr3 f AsF61-.
When H and Cl are separate the x axis the energy is at a particular value. Also there are no charges on atoms in PCl3 lewis structure. What type of Lewis structure is PCl3.
A Number of valence electrons. In PCl3 lewis structure each chlorine atom is joint with center phosphorus atom through a single bond. Pcl3 structure lewis electron dot bond phosphorus molecular geometry trichloride draw atom shape many central angles hybridization downloads.
Once we know how many valence electrons there are in PCl5 we can distribute them around the central atom and attempt to. Underneath draw the lewis structure. A SF6 b PCl5 c BeH2 d 3CH.
Pcl3 lewis structure chemistry valence electrons pcl 3k views 1e03. The straw Lewis structures for each of the following molecules for a H two is a little structure h each for be hbr. For the central atom what is the formal charge.
Science Chemistry Lewis structure. PCl3 lewis structure In this lewis structure of PCl3 center phosphorus atom has made three single bonds with three chlorine atoms. S 6 F 7 each total 48.
A single line bond represents two electronsThe total number of electrons used is 48. Draw the Lewis structure for HCN which has a triple bond. Phosphorus trichloride PCl3 contains three chlorine atoms and one phosphorus atoms.
Write Lewis structures for. Draw the Lewis structure for PCl3 phosphorus trichloride the chemical used commercially to prepare insecticides and flame retardants. Predict the electron pair geometry and the molecular structure of each of the following molecules or ions.
Write Lewis structures for the following. Percy we have PCL three. But as the difference here is more than 05 PCL3 is a polar molecule.
Best Answer 100 1 rating Previous question Next question. For the PCl5 Lewis structure we first count the valence electrons for the PCl5 molecule using the periodic table. As they approach it decreases to a minimum at 127 pm the bond distance and then it increases sharply as you get closer.
If you watch the video above and follow the steps you should end up with the following Lewis structures. H 2 CNH h NO i N 2 j CO k CN Write Lewis structures for the following. Each chlorine has 63 lone pairs.
Six bonds are formed and no nonbonded pairs exist. Next because Mg loses 2 electrons to O write Mg2 and oxygen gains two electrons so it can be drawn with eight dots surrounding it with brackets and a 2- charge. B Write the Lewis Structure to show the formation of barium nitride from barium and nitrogen.
C PCl3 d BrO21- a Write the Lewis Structure to show the formation of sodium nitride. A step-by-step explanation of how to draw the PCl3 Lewis Dot Structure Phosphorus TrichlorideFor the PCl3 structure use the periodic table to find the tot. What is the hybrid orbital designation for the central atom.
Pcl3 bond lewis structure angle bonding cl angles pairs number electron. This problem has been solved. A Write the Lewis Structure for Nitrogen.
SF two for E h 2 cc h two f h and N h yeah and double bond and H. 5 rows Chlorine has seven valence electrons but as there are three atoms of Chlorine we will multiply. There is a lone pair on center phosphorus atom and each chlorine atom also has three lone pairs.
What shape is bf3. H 3 O ceNH4 h ceBF4- i HCCH j ClCN. What is the geometric shape.
Write Lewis Structures For.

Pcl3 Lewis Structure Lewis Structure Of Pcl3 Phosphorus Trichloride Draw Lewis Structure For Pcl3 Youtube
Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization
What Is The Bond Angle Of Pcl3 Quora

Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization

Pcl3 Lewis Structure Hybridization Molecular Geometry And Mo Diagram Techiescientist

What Is The Molecular Geometry Of Pcl3 Study Com

How To Draw The Lewis Structure Of Pcl3 Phosphorus Trichloride Youtube
How To Draw Lewis Structure For Pcl3 Drawing Easy

How Is The Electron Dot Structure Of Pcl3 Determined Quora

How To Draw The Lewis Structure Of Pcl3 Phosphorus Trichloride Youtube
Is Pcl3 Non Polar Or Polar Why Quora

Pcl3 Molecular Geometry Shape And Bond Angles Youtube

Pcl3 Lewis Structure Hybridization Molecular Geometry And Mo Diagram Techiescientist

What Is The Molecular Shape Of Pcl3 Quora

Pcl3 Lewis Structure Hybridization Molecular Geometry And Mo Diagram Techiescientist

What Is The Lewis Dot Structure For Pcl3 Study Com
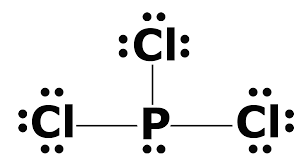
Solutions 17 Chemistry Libretexts

Hybridization Of Pcl3 Hybridization Of Phosphorus In Pcl3

Lewis Dot Structure Easy Hard Science
