Xef4 Lewis Structure Vsepr
The Lewis structure for XeF4 has a total of 36 valence electrons. Determine the Lewis structure VSEPR and the name of the shape for the following.
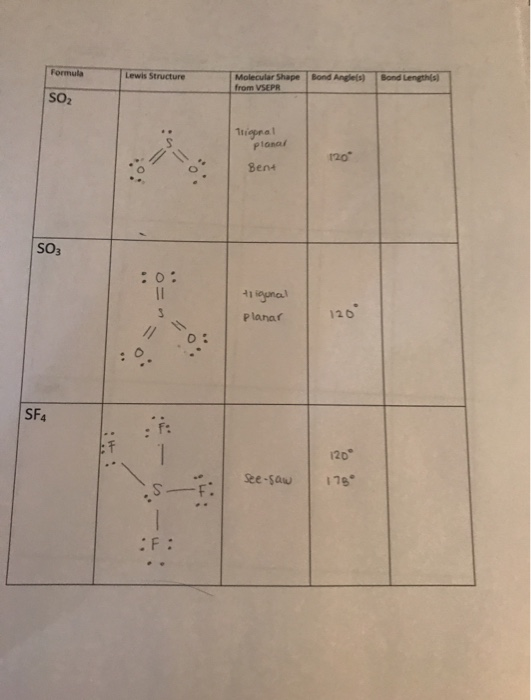
Formula Lewis Structure Molecular Shape Bond Angles Chegg Com
The shape is like a seesaw.

Xef4 lewis structure vsepr. The second step is to valence electron to the four chlorine atoms and the final step is to combine the step1 and step2 to get the SCl4 Lewis Structure. According to the VSEPR theory the shape of the molecule is predicted by the total number of electron pairs lone pairs bond pairs in the valence shell of the central Xe atom. Use lewis structure guidelinesto draw the lewis structure of XeF 4.
The Lewis structure of SF4 is the combination of 34 valence electron and 5 electron pairs around the Sulfur in which there are four bonding pairs and one lone pair. What is the vsepr structure of XeF4. Sncl3 Lewis Structure What is the molecular geometry of sncl3.
This electron arrangement is known as Trigonal Bipyramidal. This problem has been solved. Draw a Lewis structure for each of the following polyatomic ions and then use VSEPR theory to determine the geometry of each.
Indicate the best Lewis structure VSEPR structure and molecular shape of the following. The Lewis structure is shown below. B- write the VSEPR formula for XeF4.
The sublimation of the compound occurs at 1157C. Determine a valence electrons b Lewis structure c geometry and d VSEPR formula of each of the following species. BeCl2 CO2 CH2O BF3 NO2 H2O CH4 NH3 SF4 BrF3 ICl2 IF5 XeF2 PCl5.
The Lewis structure for XeF4 has a total of 36 valence electrons. Xenon Tetrafluoride XeF4 was the first discovered binary chemical compound of a noble gas Xenon. The central atom of Sn is surrounded by a pair of unbound electrons and three single bonds.
For the XeF4 Lewis structure we first count the valence electrons for the XeF4 molecule using the periodic table. A-write the lewis structure for XeF4. Applying VSEPR rules to minimise repulsion among lone pairs bond pairs lone pair-bond pairs we see that the repulsion is minimum iff lone pairs are anti to one another and the fluorine atoms occupy Equatorial position.
I NH4 ii XeF4 iii PCl5 Kaitlynn W. We must first draw the Lewis structure for XeF₂. This tells us that there are five electron regions Steric Number 5 about the central carbon atom.
Therefore the geometry of the electron pairs is tetrahedral with three of the angles occupied by the bonding electron pairs. An exothermic chemical reaction of Xenon Xe with Fluorine F leads to the production of XeF4. C-SKETCH THE 3 DIMENSIONAL STRUCTURE.
Once we know how many valence electrons there are in XeF4 we can distribute them around the central atom and attempt to fill the outer shells of each atom. What is the lewis structure Vsepr bartleby. D-what is the approximate F-Xe-F bond angles in XeF4.
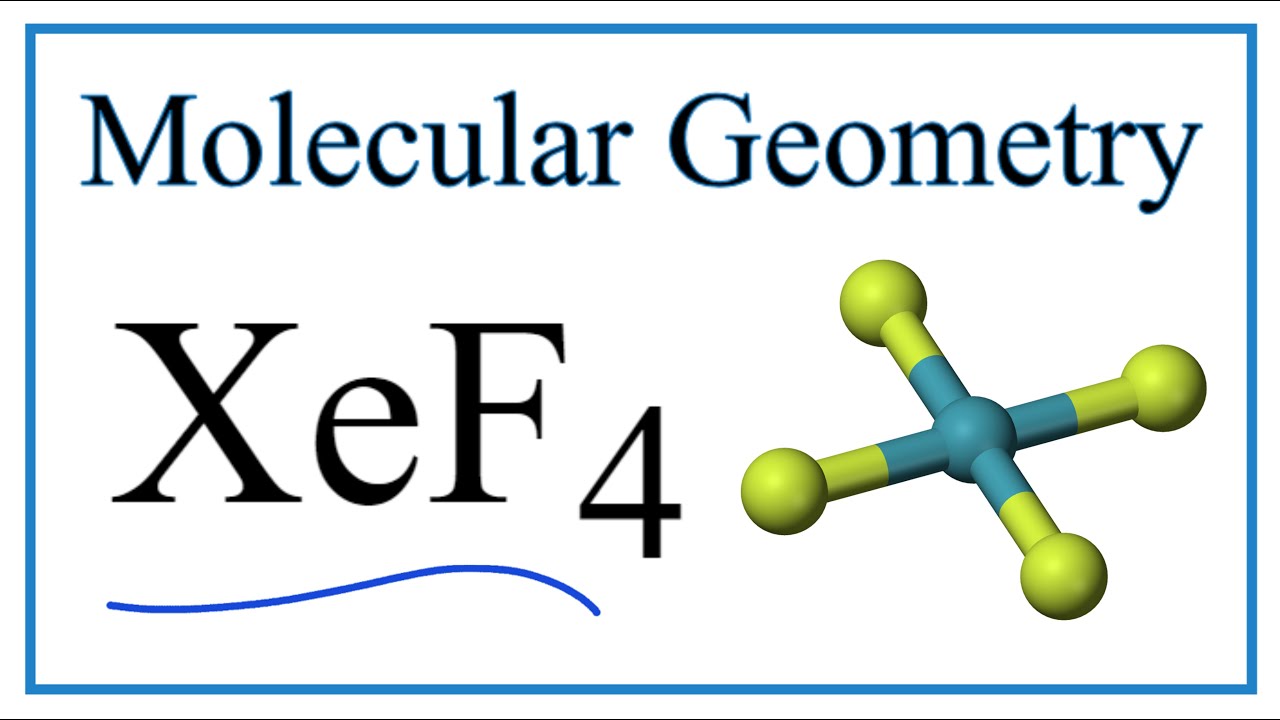
A three-step approach for drawing the SCl4 Lewis structure can be used. Lewis Structure VSEPR for XeF4 - YouTub. So the hybridization is sp3d2 and it has 2 lone pairs the shape of XeF4 is square planar.
A XeF4 b SCl2 By signing up youll get thousands. According to VSEPR theory a central atom with 4 covalently-linked terminal atoms and 2 non-bonding pairs will adopt a square planar molecular geometry shape. The first step is to sketch the Lewis structure of the SCl4 molecule to add valence electron around the sulfur atom.
AX 4 E 2 has square planar shape. Various studies regarding neutron diffraction have proved that the structure of the chemical compound is square planar. Use VSEPR table to find the shape.
We also need to check to make sure we only used the number of available valence electrons we calculated earlier no more no less. What is the lewis structure Vsepr Formula and geometric shape of these molecules. They are the three lone pairs and the two Xe-F bonds.
Key Points To Consider When Drawing The SCl4 Structure. Apply VSEPR notation A X E ANumber of central atoms XNumber of surrounding atoms E Number of lone pairs on central atom For the above molecule VSEPR notation will be AX 4 E 2. The VSEPR theory predicts that XeF₂ is linear.
XeF4 is stable when subjected to normal pressure and temperature. The same study is backed up by VSEPR theory as there are two lone pairs in xenon. SOLUTION a The Lewis structure of the SnCl3 ion looks like this.
When we are done adding valence electrons we check each atom to see if it has an octet full outer shell. Central atom Xenon in XeF4 is sp3d2 hybridised suggesting that the gross geometry should be Octahedral.
Does The Vsepr Theory Predict That Xef2 Is Linear Socratic
What Is The Vsepr Structure Of Xef4 Quora

What Is The Vsepr Structure Of Xef4 Quora
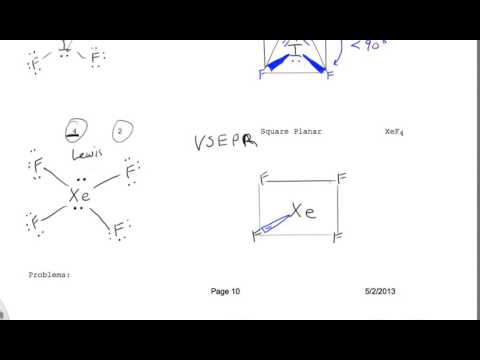
Vsepr Drawing For Xef4 Square Pyramidal Youtube
What Is The Vsepr Structure Of Xef4 Quora

Science Coverage Is No2 Polar Or Nonpolar Electron Affinity Covalent Bonding Molecular Geometry

Vsepr Theory Vsepr Theory Organic Chemistry Chemistry

Valence Shell Electron Pair Repulsion Vsepr Chemogenesis Electrons Shells Pairs
Xef2 Lewis Structure Polarity Hybridization And Shape
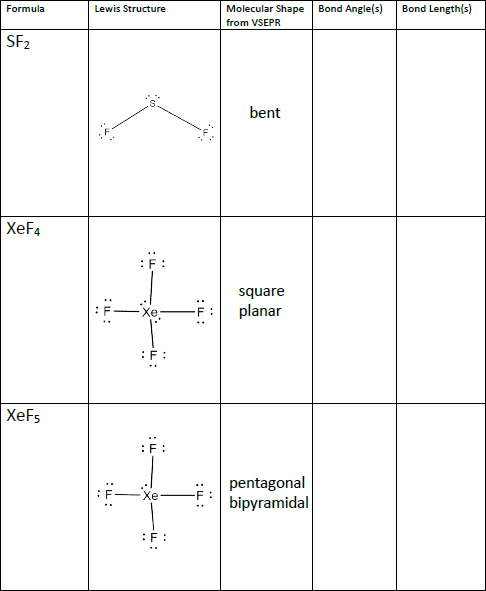
Formula Lewis Structure Bond Angle S Molecular Shape Chegg Com

Science Coverage Is Asf5 Polar Or Nonpolar In 2021 Molecular Geometry Molar Mass Octet Rule

Science Coverage Is Asf3 Polar Or Nonpolar In 2021 Molecular Geometry Polar Octet Rule

Lewis Structure Vsepr For Xef4 Youtube

What Is The Vsepr Structure Of Xef4 Quora
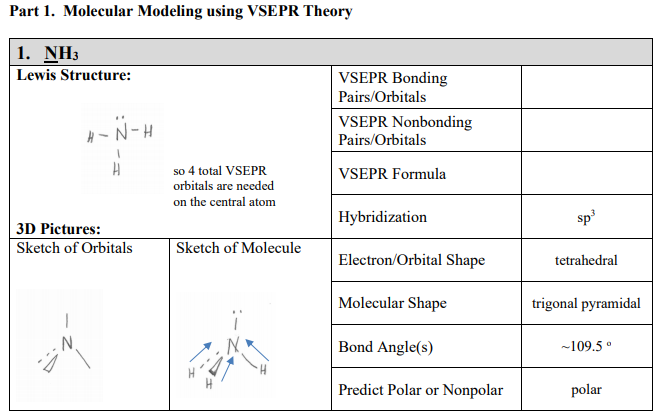
Part 1 Molecular Modeling Using Vsepr Theory 1 Nh3 Chegg Com

Ch2o Lewis Structure Methanal Or Formaldehyde In 2021 Methanal Molecules Lewis

Vsepr For 4 Electron Clouds Video Khan Academy
Xef4 Molecular Geometry Bond Angles Electron Geometry Youtube