Best Lewis Structure For Xef4
Chances of scoring your very best at exam time U Can. In the best Lewis structure for XeF4what is the formal charge on the F atom.
This rule is not law and has various exceptions.
Best lewis structure for xef4. O square planar tetrahedral O seesaw O bent Question 25 Which of the following isare polar. How_to_draw_the_lewis_dot_structure_for_xef4 25 How To Draw The Lewis Dot Structure For Xef4. The Practical Science makes the connections from.
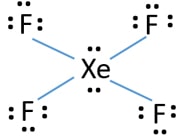
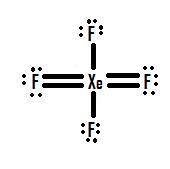
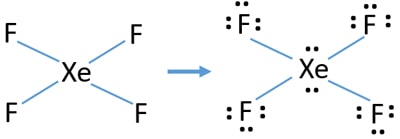
The Lewis structure for XeF4 has a total of 36 valence electrons. Well we got 8 4 7 36 electrons ie. The Lewis Structure for this molecule is.
For the Lewis structure for SF4 you should take formal charges into account to find the best Lewis structure. The Lewis structure for XeF4 is a bit tougher since you have to take formal charges into account to find the best Lewis structure for the molecule. In this tutorial we will learn how to draw lewis structure of XeF 4 step by step.
This Lewis dot structure is a pictorial representation of valence electrons around individual atoms in a molecule along with the bond it forms. Xenon is an inert gas element. 18 electron pairs to distribute over 5 centres Now FOUR of the electron pairs constitute the X e F bonds and EACH fluorine bears 3 non-bonding lone pairsand so TWO lone.
This is particularly explained by the valence bond theory as the two non-bonding electrons from 5p are promoted to the 5d orbital. Well we got math84736math electrons ie. In the best Lewis structure for XeF4 what is the formal charge on the F atom.
Become a member and unlock all Study. CBr2H2 BH3 XeCl4 SF4 HCl. Each fluorine atom has three lone pairs.
A step-by-step explanation of how to draw the XeF4 Lewis Dot Structure Xeon TetrafluorideFor the XeF4 structure use the periodic table to find the total n. Chemistry questions and answers. The octet rule states that atoms want to fill their outer valence shell with 8 electrons this molecule defies this rule because in seeking a formal charge of 0 Xe accumulates 12 total electrons.
Lewis Structure also known as electron dot structure is an essential model of chemical bonding where we use the valence electron concept to schematically sketch a two-dimensional figure of a given molecule. Draw the Lewis structure for i CO ii XeF4 iii PCl3 By signing up youll get thousands of step-by-step solutions to your homework. In XeF 4 Xenon tetrafluoride lewis structure there are four sigma bonds and two lone pairs around xenon atom.
Identify the geometry of XeCl4. Each fluorine atom has three lone pairs. Kelter 2008-01-01 From core concepts to current applications Chemistry.
To build the Lewis structure we must first determine the valence electrons of Xenon tetrafluoride XeF4 molecule as well as the atom location in the molecule. In order to calculate the formal charges for XeF4 well use the equationFormal charge of valence electrons - nonbonding val electrons - bonding ele. Math18math electron pairs to distribute over 5 centres Now FOUR of the electron pairs constitute the mathXe-Fmath bonds and EACH fluorine bears 3 non-bonding lone pairsand so TWO lone.
The shape of the molecule is a distorted trigonal bipyramid with the oxygen found on the equator. In the best Lewis structure for XeF4what is the formal charge on the F atom. Note that Sulfur is the least electronegative element in the SF4 Lewis structure and therefore goes in the middle of the structure.
In the XeF4 MO diagram it is quite clear that the structure of the compound is square planar. We use dots to represent outer shell electrons and lines to represent the bond type. We also need to check to make sure we only used the number of available valence electrons we calculated earlier no more no less.
The octet rule states that atoms seek to surrounded by 8 electrons each to fill their valence shells. XeF4 Lewis Structure Now that we know the valence electrons of Xenon Tetrafluoride it will be easier for you to draw its Lewis structure. So the hybridization is sp3d2 and it has 2 lone pairs the shape of XeF4 is square planar.
It has a distance of 195 A between the Xe and F. 6 rows For the XeF4 Lewis structure we first count the valence electrons for the XeF4 molecule. Chemistry I For Dummies shows you that you can.
There are a total of 34 valence electrons in the Lewis structure for SF4. What is the Lewis structure for XeF4. When we are done adding valence electrons we check each atom to see if it has an octet full outer shell.
In the molecular geometry of the XeF4 molecule the Xenon Xe atom in the center of the square planar geometry and all four fluorine atoms at the corner of the square planar geometry. XeF2 Lewis Structure. What is the structure of osf4.
Question 23 Which relates to the actual shape of the bonds in the compound. B-1 C 42 D 0 A 1. Molecular geometry o electronic geometry Draw the best Lewis dot structure for XeF4.
See full answer below. What is its molecular geometry. The formal charge of an atom in a molecule refers to the charge assigned to.
5 5 Violations Of The Octet Rule Chemistry Libretexts

How To Draw Xef4 Lewis Structure Science Education And Tutorials
Xef4 Xenon Tetrafluoride Lewis Structure
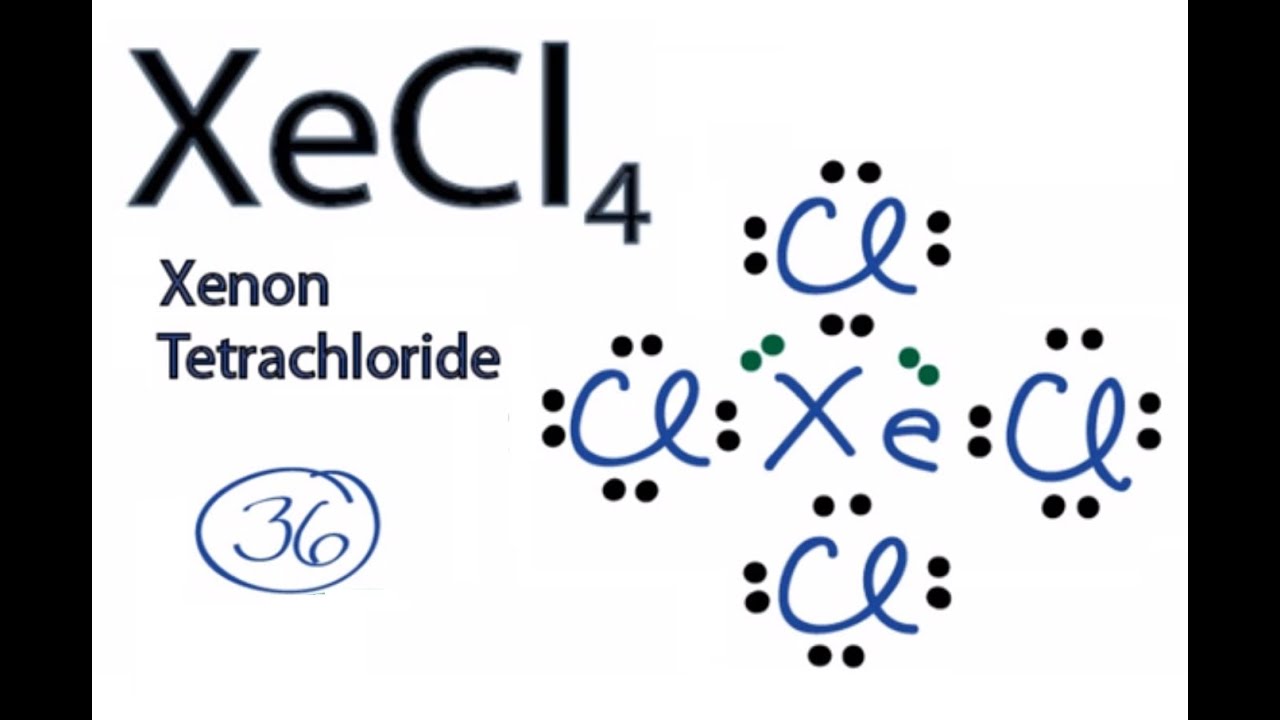
Xecl4 Lewis Structure How To Draw The Lewis Structure For Xecl4 Xenon Tetrachloride Youtube

Xef4 Lewis Structure How To Draw The Lewis Structure For Xef4 Youtube

How To Calculate The Formal Charges For Xef4 Xenon Tetrafluoride Youtube
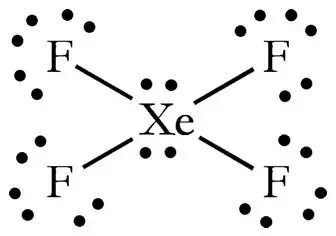
How Can The Lewis Structure For Xef4 Be Determined Quora

Xef6 Lewis Structure How To Draw The Lewis Structure For Xef6 Youtube

Xef4 Molecular Geometry Bond Angles Electron Geometry Youtube

Best Overview On Is Xef4 Polar Or Nonpolar Science Education And Tutorials

Answer The Questions In The Table Below About The Shape Of T Clutch Prep
How Can The Lewis Structure For Xef4 Be Determined Quora

Xef4 Xenon Tetrafluoride Lewis Structure

Xef4 Lewis Structure How To Draw The Lewis Structure For Xef4 Youtube

What Is The Vsepr Structure Of Xef4 Quora
Xef4 Xenon Tetrafluoride Molecular Geometry Lewis Structure And Polarity Geometry Of Molecules
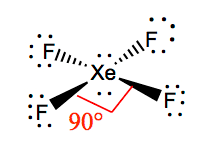
Xef4 Xenon Tetrafluoride Molecular Geometry Lewis Structure And Polarity Geometry Of Molecules

In The Best Lewis Structure For Xef4 What Is The Formal Charge On The F Atom A 1 B 0 C 1 D 2 Study Com
