Bf3 Lewis Structure Double Bond
If it is in the form of a colorless liquid it is very soluble dihydrate. What is the Lewis dot structure of BF3.
Bf3 Lewis Structure Molecular Geometry Hybridization And Polarity
Boron is the least electronegative atom in the BF3 Lewis.

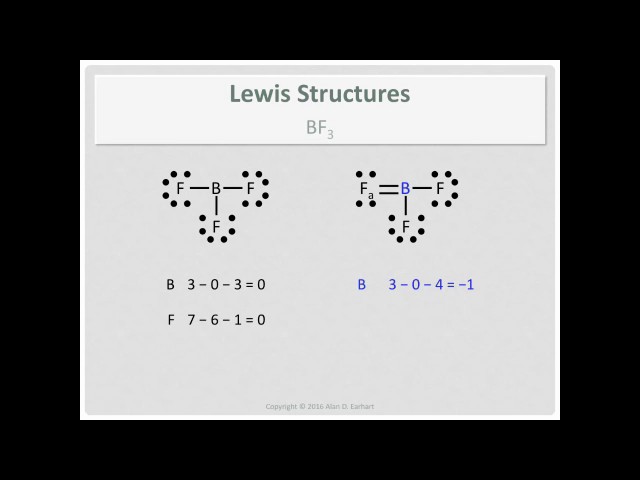
Bf3 lewis structure double bond. Each O is surrounded by four dots and two sticks or lines representing another 4 electrons in the O2 double bond. For this molecule It is SP2 because one π pi bond is required for the double bond between the Boron and only three σ bonds are formed per Boron atom. If3 Lewis Structure For the BF3 Lewis structure calculate the total number of valence electrons for the BF3 molecule.
So each O is surrounded by 8 total valence electrons giving it an octet and making it stable. Because it takes six electrons to form the skeleton structure there are 18 nonbonding valence. After determining how many valence electrons there are in BF3 place them around the central atom to complete the octets.
BrF3 Lewis Structure Molecular Geometry Hybridization and MO Diagram. Using our rules for drawing Lewis dot structures we complete the octet around the B by forming double bonds from one of the F atoms which give rise to the resonance structures shown in Structure B. 3 37 24 There are three covalent bonds in the most reasonable skeleton structure for the molecule.
BF3 is SP2 hybridization. The correct Lewis structure for BF3 would have exactly. Lewis structures for NH3 and BF3.
What is the correct name for BF3. What is the Lewis dot structure for oxygen. Structural characterization of BF 3 suggests that there are no double bonds to F and that Structure A is the actual structure even though is does not obey the octet rule.
None of the above. The covalent bond tells us that electrons are shared rather than lost by boron and gained by fluorine. A Lewis structure for BF3Chem 1090 Lewis 7b.
There are a total of 24 valence electrons for the BF3 Lewis structure. Due to the resonance structure of the BF3 molecule creates a double bond and a single bond. Question 3 3 pts A 500 liter balloon of gas at 25C is cooled to 0C.
The two letter Os in the O2 Lewis structure represent the nuclei centers of the oxygen. The double bond of B-F is shorter as compared to the single bond of B-F. Lewis Structure for BF3 Boron Trifluoride.
Which concludes option E as correct. BrF3 known as Bromine Trifluoride is a fuming liquid consisting of inter-halogen combinations and bearing a pungent smell. According to the Lewis structure of BF 3 boron in the center forms three bonds which Florine atom.
There are a total of 24 valence electrons for the BF3 Lewis structure. Having a straw ie colorless to yellow appearance this chemical compound has several applications but also comes with a number of limitations and hazard issues. What type of bond is BF3.
Group of answer choices. Question 2 3 pts The correct Lewis structure for BF3 would have exactly. BF3 is a molecule consisting of an sp2 hybrid of Boron covalently bonded with 3 atoms of fluorine.
Boron is the least electronegative atom in the BF3 Lewis structure and therefore goes at the center of the structure. This bond is formed because of Borons high ionization energy. Total24 Hybridization stands for mixing atomic orbitals into new hybrid orbitals.
After determining how many valence electrons there are in BF3 place them around the central atom to complete the octets. Boron has atomic number 5 and it has three valence electron on the other hand fluorine atom need one electron to complete its octet therefore boron form three covalent bond single bond with three fluorine atom. BF3 is SP2 hybridization.
BF3. Structural characterization of BF 3 suggests that there are no double bonds to F and that Structure A is the actual structure even though is does not obey the octet rule. The atomic S orbitals and P orbitals in Boron outer shell mix to form three equivalent SP2 hybrid orbitals.
The B-F bond length of the double bond is 130 pmpicometer. In this case we can justify this structure. Question 2 3 pts The correct Lewis structure for BF3 would have exactly.
The valency of B Boron is 3 and of F Fluorine is 7 thus the Lewis structure of BF3 can be drawn as shown in the figure.
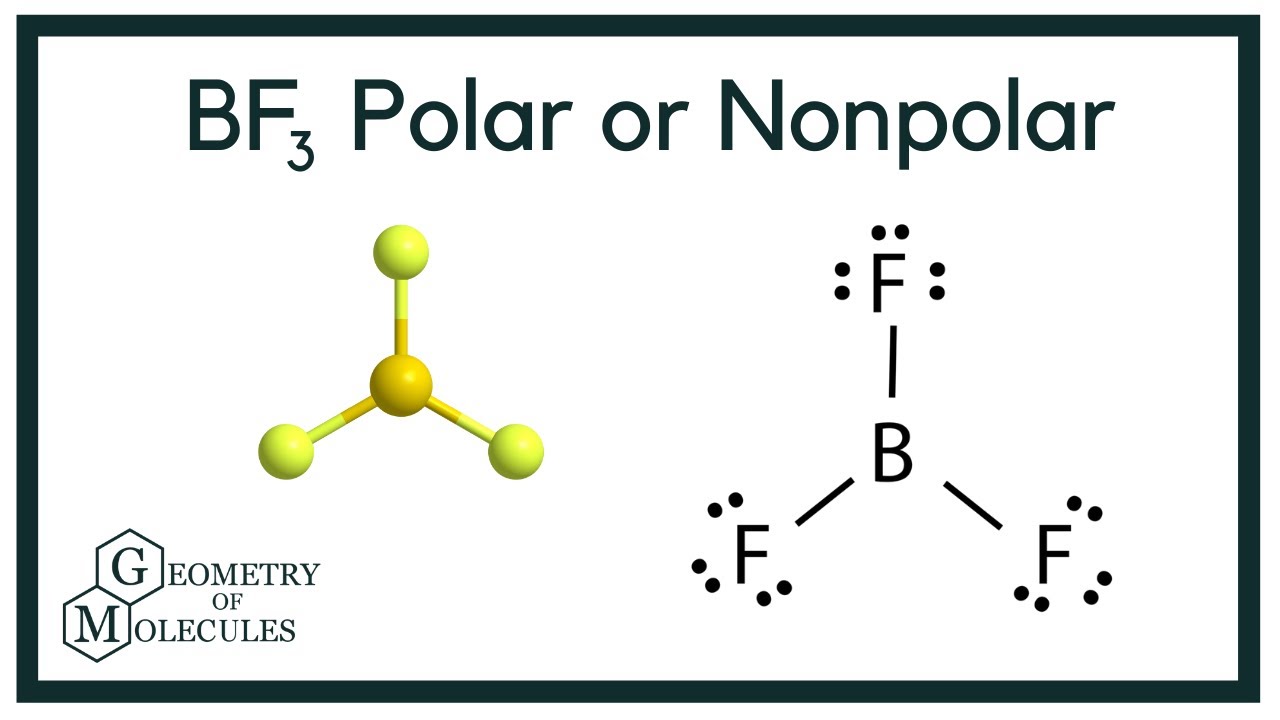
Bf3 Lewis Structure Molecular Geometry Hybridization And Polarity

2 Consider The Molecule Bf3 See Section 5 2 A Chegg Com
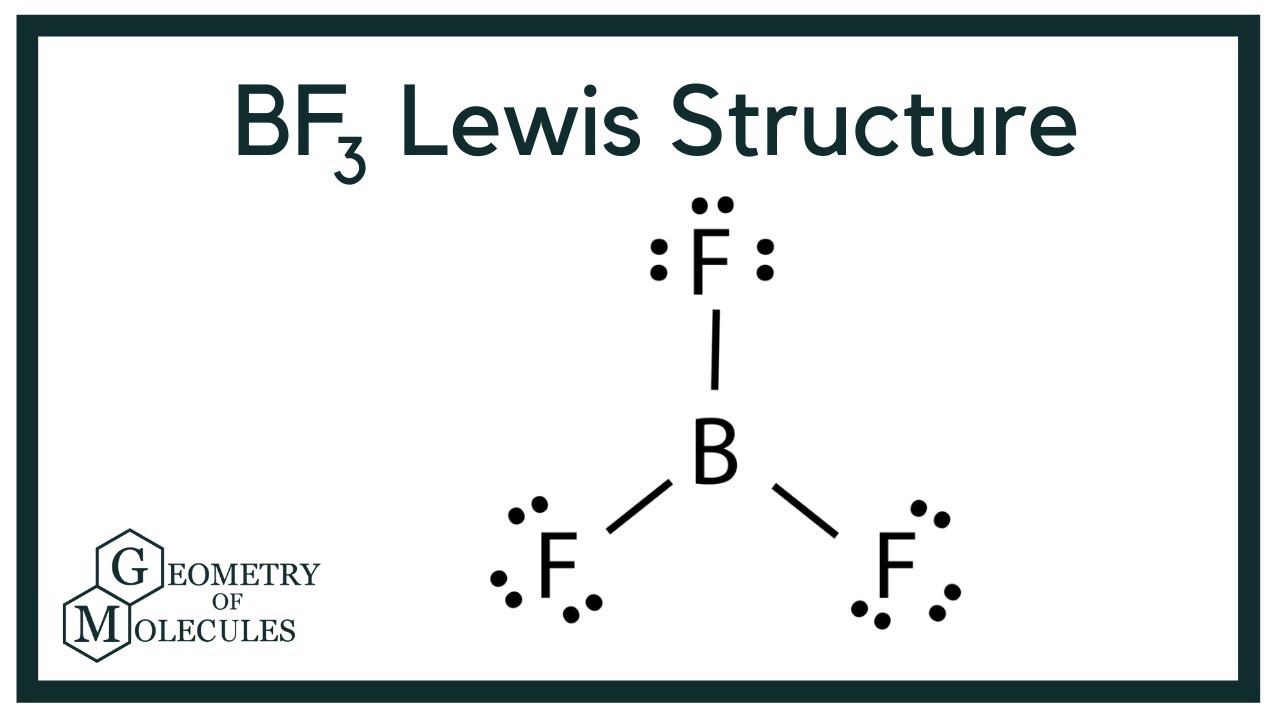
Bf3 Lewis Structure Molecular Geometry Hybridization And Polarity

Solution For The Molecule Bf3 If We Wri Clutch Prep
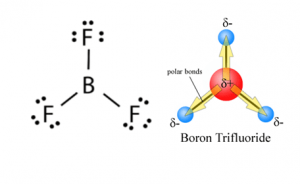
Bf3 Lewis Structure Molecular Geometry Hybridization And Polarity

Solution For The Molecule Bf3 If We Wri Clutch Prep

Bf3 Lewis Structure How To Draw The Lewis Structure For Bf3 Youtube

Why Is Bf3 Written In Two Ways Quora

Why Is Bf3 Written In Two Ways Quora

Bf3 Lewis Structure How To Draw The Lewis Structure For Bf3 Youtube

Why Is Bf3 Written In Two Ways Quora
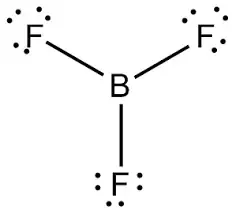
Why Is Bf3 Written In Two Ways Quora

Give The Lewis Dot Structure Of Bf3 Study Com
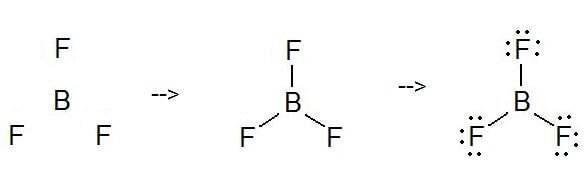
Bf3 Lewis Structure Molecular Geometry Hybridization And Polarity

Bf3 Lewis Structure Molecular Geometry Hybridization And Polarity

9 3 Drawing Lewis Structures Chemistry Libretexts
Why Is Bf3 Written In Two Ways Quora