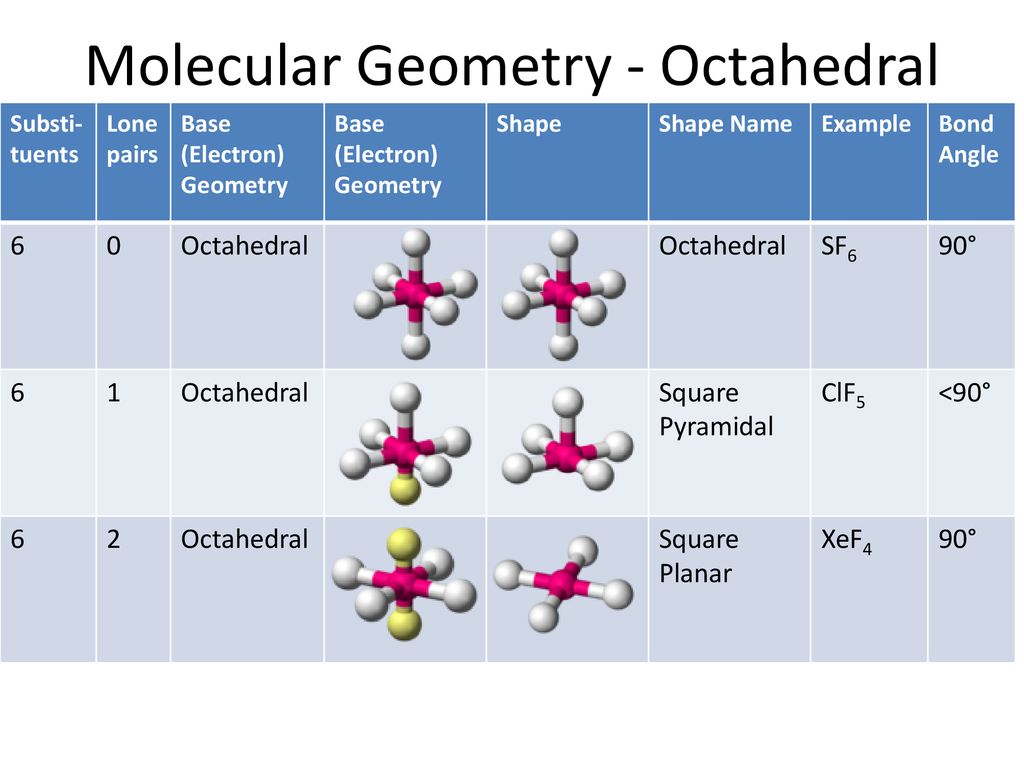
Clf5 Molecular Shape
Since chlorine has 7 electrons in the valence shell the remaining two electrons form a lone pair. As per the VSEPR theory valence shell electron pair repulsion theory the shape of a ClF5 molecule is square pyramidal.
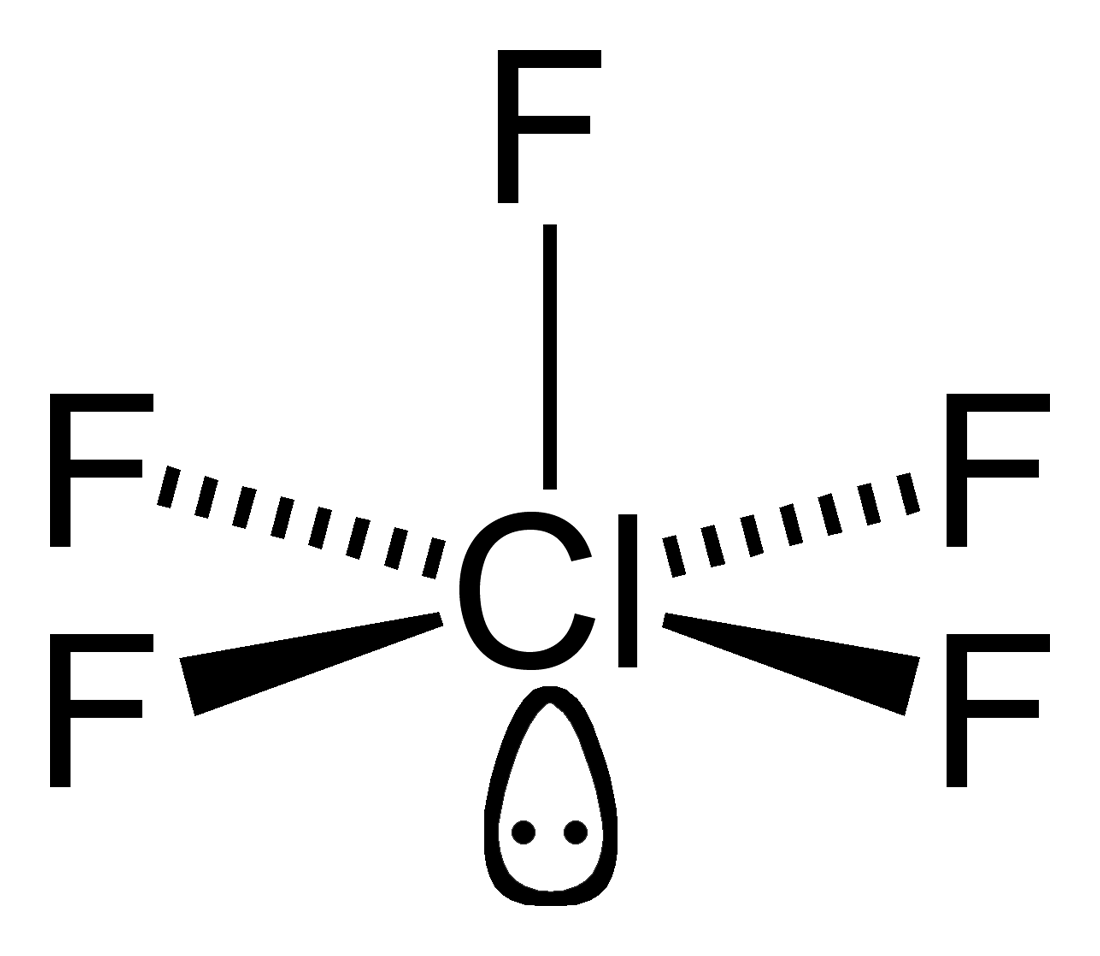
File Chlorine Pentafluoride 2d Lone Pair Png Wikimedia Commons
Presearch is a decentralized search engine powered by the community.

Clf5 molecular shape. In a ClF5 molecule chlorine is the central atom featuring five single bonds with fluorine atoms. Also N in NH3 is sp3 hybridised so original shape of NH3 molecule should be tetrahedral but because of the lone pair it is pyramidal in shape. Shapes of molecules VSEPR.
What is the molecular geometry of. SF 6 Sulfur Hexafluoride. As per the VSEPR theory valence shell electron pair repulsion theory the shape of a ClF5 molecule is square pyramidal.
ChemDoodle What is the electron-pair geometry for. What is the molecular geometry of ClF5. In a ClF5 molecule chlorine is the central atom featuring five single bonds with fluorine atoms.
Chlorine pentafluoride ClF5 CID 61654 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information. Back to Molecular Geometries Polarity Tutorial. In a ClF5 molecule chlorine is the central atom featuring five single bonds with fluorine atoms.
NH3 has 3 bond pairs and 1 lone pair. SF 4 Sulfur Tetrafluoride. The molecular geometry of ClF 5 is square pyramidal with asymmetric charge distribution around the central atom.
Since chlorine has 7 electrons in the valence shell the remaining two electrons form a lone pair. ClF5 Molecular Geometry 76 Molecular Structure and Polarity Chemistry molecular geometry structure chemistry polar nonpolar lone pairs electron pair shape vsepr table molecules polarity vs bonded chem bonding trigonal. What is the electron-pair geometry for Cl in ClF5.
As per the VSEPR theory valence shell electron pair repulsion theory the shape of a ClF5 molecule is square pyramidal. As per the VSEPR theory valence shell electron pair repulsion theory the shape of. So the original shape of the compound should have been square bipyramidal but due to the presence of 1 lone pair the shape is square pyramidal.
Also Cl in ClF5 is sp3d2 hybridised. The molecular geometry of ClF 5 is square pyramidal. The general form of the molecule is AB5.
The molecule has 42 electrons in the valence orbitals of the 6 atoms. From Wikipedia the free encyclopedia Chlorine pentafluoride is an interhalogen compound with formula ClF 5. Organometallics Metal-Ligand Bonding Carbon Monoxide.
Since chlorine has 7 electrons in the valence shell the remaining two electrons form a lone pair. On the periodic table its in group 7 or 17 so it has 7 valence electrons. The molecule adopts a square pyramidal structure with C 4v symmetry as confirmed by its high-resolution 19 F NMR spectrum.
In a ClF5 molecule chlorine is the central atom featuring five single bonds with fluorine atoms. Since chlorine has 7 electrons in the valence shell the remaining two electrons form a lone pair. PF 5 Phosphorus Pentafluoride.
Chlorine Pentafluoride on Wikipedia. What is the the shape molecular geometry of CIF5. ClF 3 Chlorine Trifluoride.
In a ClF5 molecule chlorine is the central atom featuring five single bonds with fluorine atoms. The molecules consists of five single bonds each connecting a fluorine to the central Cl and one. H 2 O Water.
Therefore this molecule is polar. Similarly ClF5 has 5 bond pairs and 1 lone pair. XeF 4 ClF 3 and CCl 3 Br.
The molecular geometry of ClF5 is square pyramidal with asymmetric charge distribution around the central atom. Molecular Geometry Polarity Tutorial. Since chlorine has 7 electrons in the valence shell the remaining two electrons form a lone pair.
As per the VSEPR theory valence shell electron pair repulsion theory the shape of a ClF5 molecule is square pyramidal. This colourless gas is a strong oxidant that was once a candidate oxidizer for rockets.

Clf5 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Vsepr Theory And Polar Molecules Ppt Download

Clf5 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Chlorine Pentafluoride Clf5 Lewis Structure Lewis Structure For Brf5 Molecular Geometry Bond Angle Hybridization Polar Or Nonpolar Just Another
Neet Ug Hybridization In Sf4 Clf5 Carbonate Nitrate Ammonium Ion Offered By Unacademy

Clf5 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Ignoring Lone Pair Effect What Is The Smallest Bond Angle I Clutch Prep

Vsepr The Familiar Vsepr Valence Shell Electron Pair Repulsion Approach To Molecular Structure Was Developed By Ronald Gillespie The Basic Idea Is Ppt Download
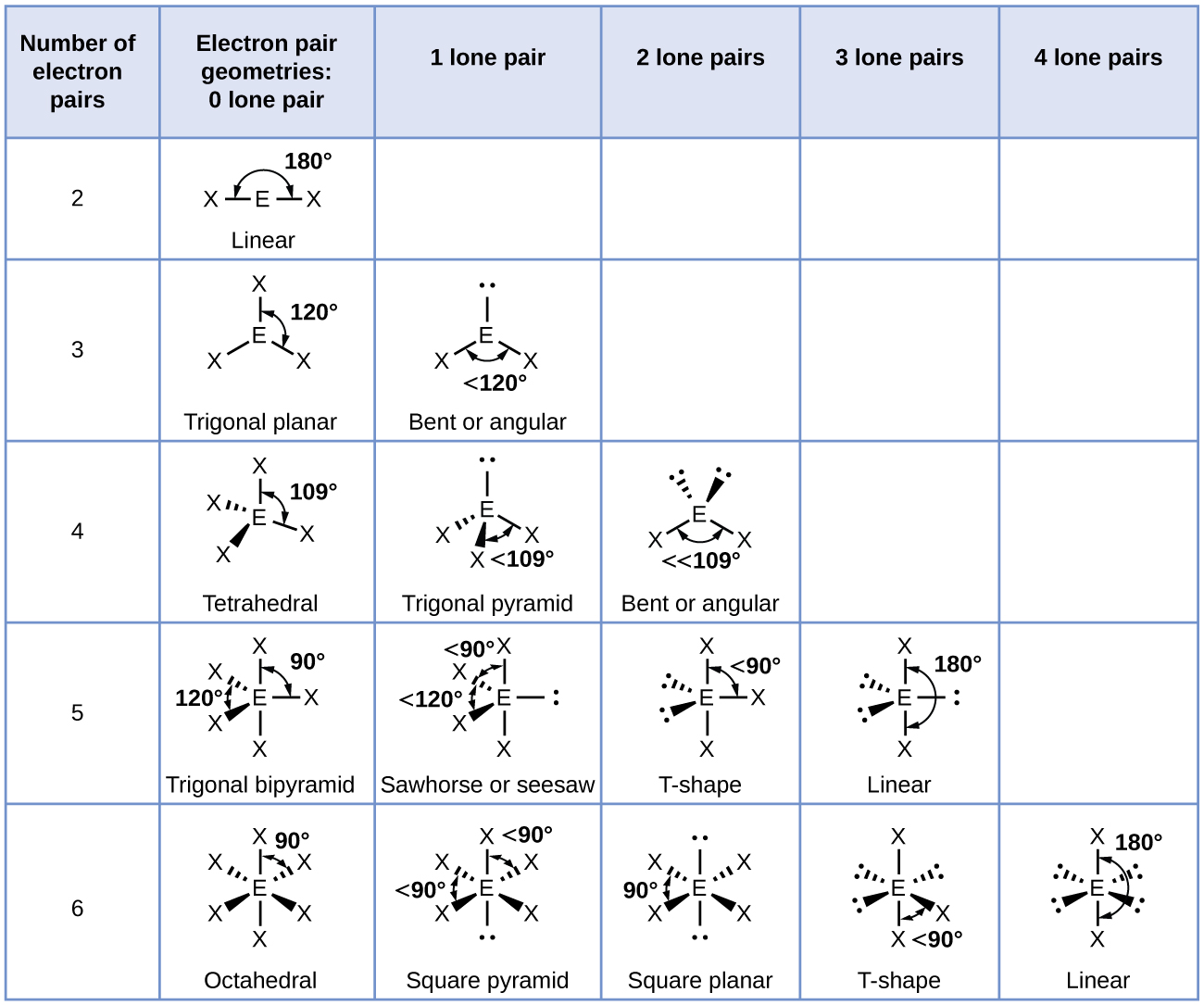
7 6 Molecular Structure And Polarity Chemistry
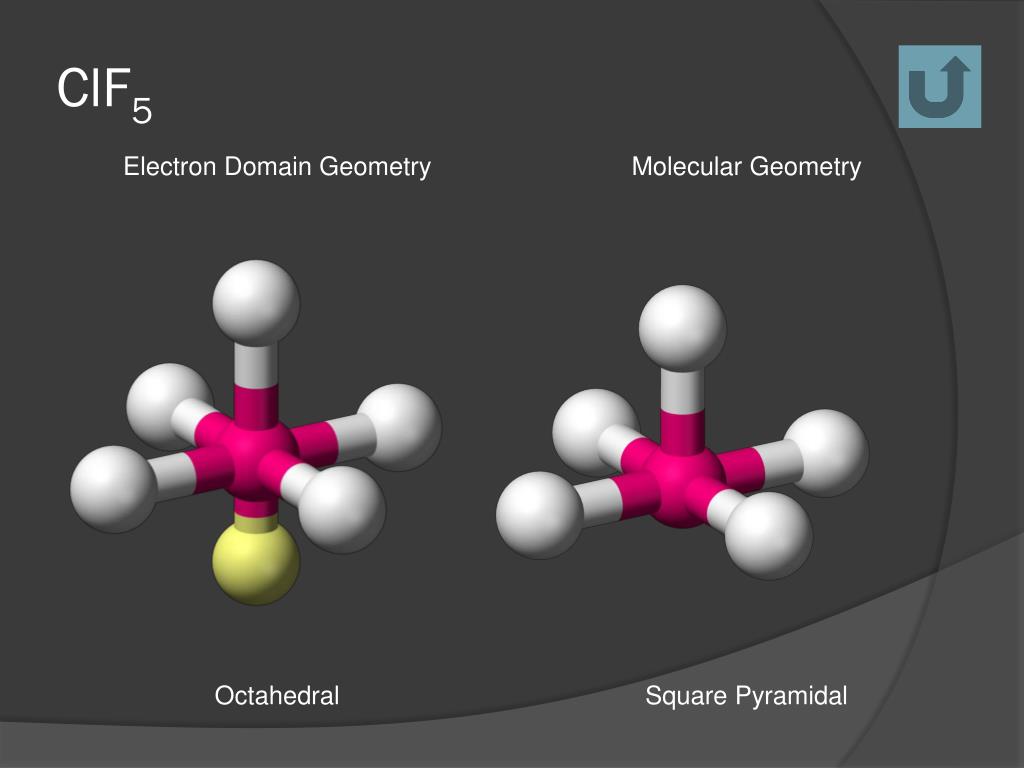
Ppt Molecular Geometry Powerpoint Presentation Free Download Id 3887508
Is Clf5 Polar Or Non Polar Quora

Molecular Structure And Polarity Ns 104 General Chemistry 1 Svc Openstax Cnx
Chapter 8 Covalent Compounds Bonding Theories And Molecular Structure

Clf5 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist
2 10 16 Today I Will Determine The Shapes Of Small Molecules Ppt Download

Vsepr Molecular Geometry Example 2 Clf5 Youtube
What Is The Shape Of Clf5 Using Vsepr Theory Quora