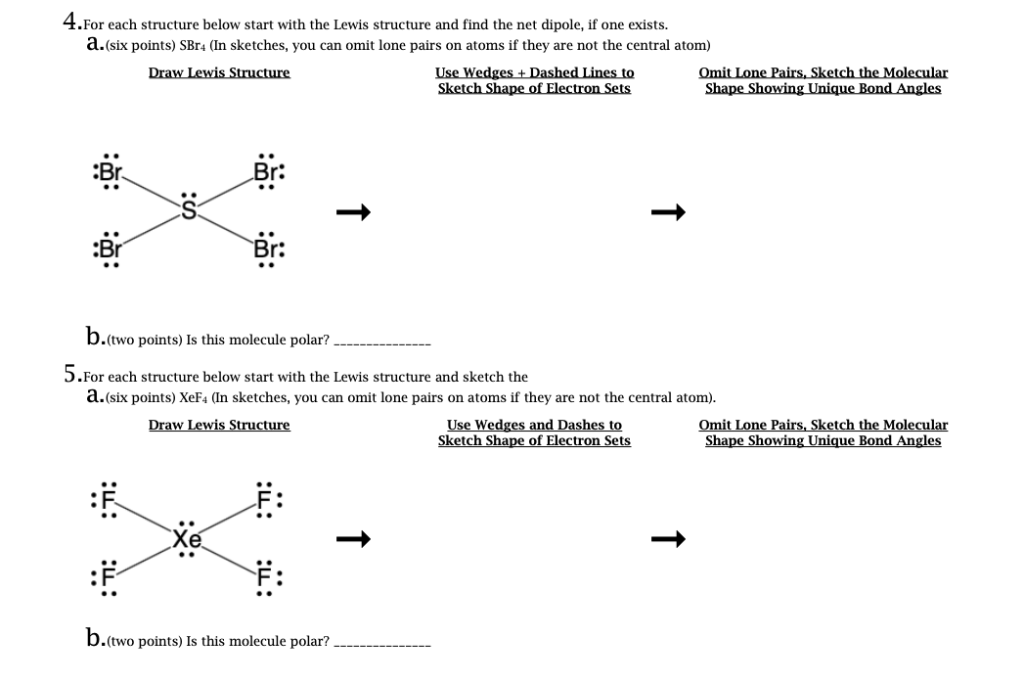
Draw The Electron-dot Structure For Chclo. Carbon Is The Central Atom
Draw the electron-dot structure for chclo. Carbon is the central atom and all three atoms H Cl O are attached to the carbonDraw the molecule by placing the atoms on the grid and connecting them with bonds.

Draw The Electron Dot Structure For Chclo Carbon Is The Cen Clutch Prep
Draw the electron-dot structure for chclo.

Draw the electron-dot structure for chclo. carbon is the central atom. A step-by-step explanation of how to draw the CHClO Lewis Dot StructureFor the CHClO Lewis structure calculate the total number of valence electrons for th. Draw the electron-dot structure for CHClO. The hydrogen oxygen and chlorine are attached to it.
Electron-dot structure for CHClO. Draw the electron-dot structure for CHClO. Draw the molecule by placing the atoms on the grid and connecting them with bonds.
Include all lone pairs of electrons. In CHClO there are 18 valence. Draw the electron-dot structure for CHClO.
O prefers 2 bonds. The Lewis structure for CHClO requires you have a double between the Carbon C and Oxygen O atoms in order to fill the octets on the Carbon. It is also known as Lewis-dot structure.
CHClO carbon is the central atom. Include all lone pairs of electrons. Carbon is the central atom and all three atoms H Cl O are attached to the carbon.
Public health information CDC Research information NIH. Carbon is the central atom and all three atoms H Cl O are attached to the carbon. Draw the electron-dot structure for CHClO.
Thus the ion will be polar unless the Lewis. A step-by-step explanation of how to draw the CHCl3 Lewis Dot Structure ChloroformFor the. In the given compound formyl chloride carbon is present as a central atom.
Draw The Lewis Structure For CHClO. Why or why not. Dots are just spacers.
C prefers 4 bonds. C4H6O2 a Draw a plausible structure for this compound that contains alcohol ether and alkyne functional groups. Include all lone pairs of electrons.
Draw the molecule by placing the atoms on the grid and connecting them with bonds. Draw the electron-dot structure for CHClO. Carbon is the central atom and all three atoms H Cl O are attached to the carbon.
B Draw a plausible structure for this compound that contains. Identify the number of hydrogen atoms bound to each carbon in the structure. For Cl add 2 dots to right left and bottom.
Drawing a lewis structure is the first step to determine bond angles. Therefore each P-O bond is somewhat polar. Include all lone pairs.
A step-by-step explanation of how to draw the CHClO Lewis Dot StructureFor the CHClO. Carbon is the central atom and all three atoms H Cl O are attached to the carbon. A step-by-step explanation of how to draw the CCl4 Lewis Dot Structure Carbon tetrachloride.
Draw the electron-dot structure for CHClO. I am asked to draw the dot and cross diagram to. C is the central atom.
Carbon is single bonded to H and Cl and double bonded to O. Draw the molecule by placing the atoms on the grid and connecting them with bonds. Include all lone pairs of electrons.
Three lone pairs of electrons on Cl and two lone pairs on O. Is PO43- polar or non-polar. Draw the skeletal structure line-angle line-bond mode of 2-isopropyltoluene.
Draw an appropriate Lewis structure for the following compounds. Include all lone pairs of electrons. There are 18 valence electrons for the CHClO Lewis structure.
Draw The Electron Dot Structure For Chclo Easy Drawing Draw The Electron Dot Structure For Chclo. Look up the electronegativity of P and O. 1 C is the central atom.
Carbon is the central atom and all three atoms H Cl O are attached to the carbon. Include all lone pairs of electrons. The Lewis structure for CCl4 is a commonly tested Lewis struc.
It shows the bonding between the atoms of a molecule and it also shows the unpaired electrons present in the molecule. Draw the molecule by placing the atoms on the grid and connecting them with. The valence electrons are represented by dotThe given molecule is As we know that carbon has 4 valence electrons hydrogen has 1 valence electron chlorine has 7 valence electrons and oxygen has 6 valence electronsTherefore the total number of valence electrons.
I think I remember that O is about 35 and P is about 21. Carbon is the least electronegative atom and goes in the center of this structure. Cl prefer 1 bond.
Draw the electron-dot structure for C H C l O. Carbon is the central atom. Answer Electron-dot structure.
The carbon has four valance electrons the hydrogen atom has one. Draw the molecule by placing the atoms on the grid and connecting them with bonds.

Part A Draw The Lewis Structure For Chclo Clutch Prep

Based On Your Answers To Parts A B And C Clutch Prep

Draw The Lewis Structure Of The Following Clutch Prep

Solution Draw The Lewis Dot Structure Fo Chemistry

Solution Draw The Lewis Dot Structure Fo Chemistry

Cncl Lewis Structure How To Draw The Lewis Structure For Cncl Youtube

Based On Your Answers To Parts A B And C Clutch Prep

Draw The Electron Dot Structure For Chclo Clutch Prep

Formal Charges Calculating Formal Charge Youtube

Draw The Electron Dot Structure For Chclo Clutch Prep

Draw The Lewis Structure For Chclo Draw The Molecule By Pla Clutch Prep

Lewis Dot Structures Glad Teaching Chemistry Chemistry Lessons Chemistry Classroom

Chclo Lewis Structure How To Draw The Lewis Structure For Chclo Youtube

Draw The Electron Dot Structure For Chclo Carbon Is The Cen Clutch Prep
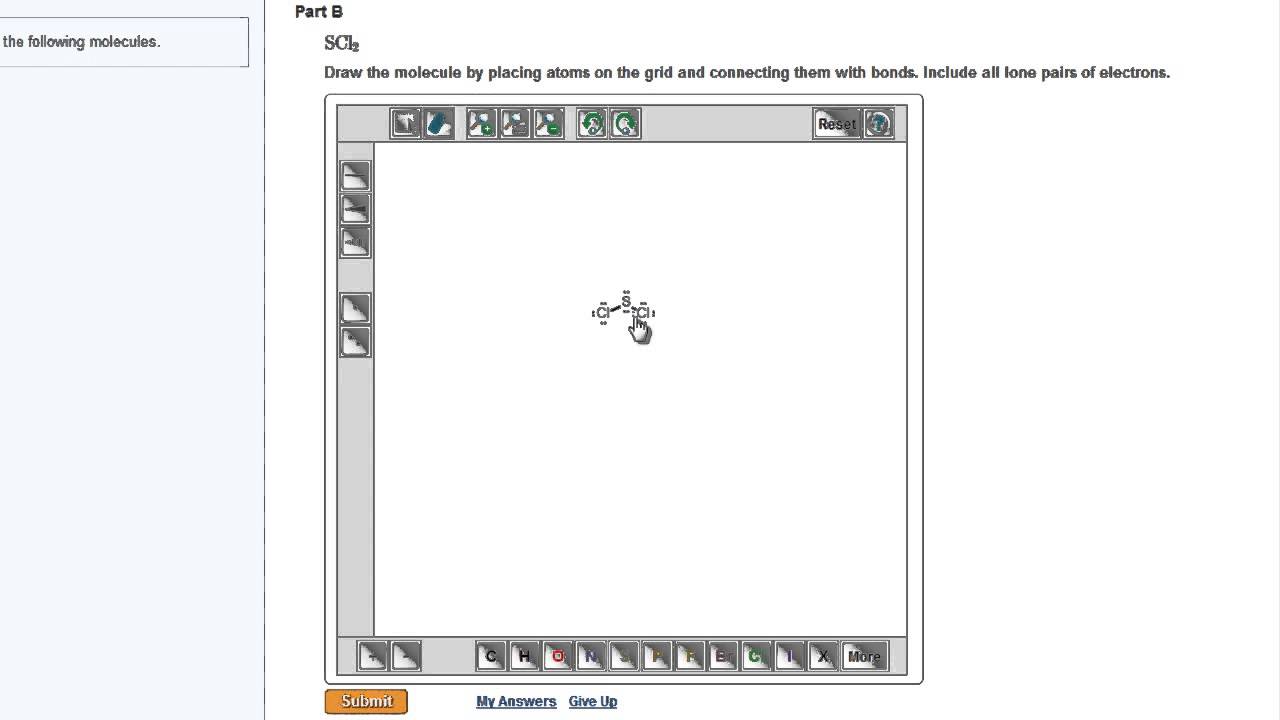
Masteringchemistry Drawing Lewis Structures Youtube