Draw A Lewis Structure For Co2
So we have 12 plus 4 16 total valence electrons. Draw Lewis Structure CO2 images similar and related articles aggregated throughout the Internet.

Co2 Lewis Structure Easy Hard Science
Here are the steps that I follow when drawing a Lewis structure.

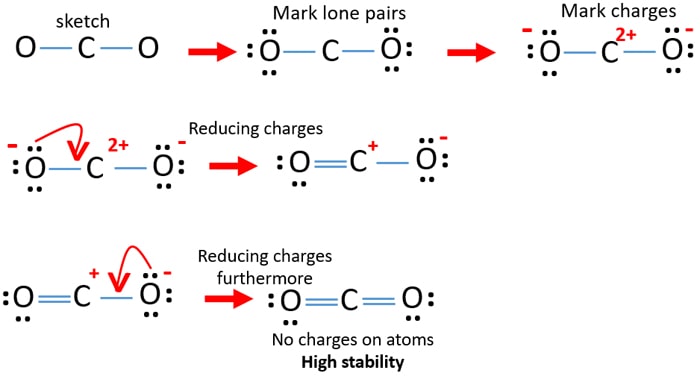
Draw a lewis structure for co2. You could alternatively also draw the structure by including two dots for every bond. Steps of drawing lewis structure of CO 2 Determine total number of electrons of the valance shells of carbon and oxygen atoms Total electrons pairs existing as lone pairs and bonds Determine center atom and drawing the sketch Mark lone pairs on atoms Mark charges on atoms if there are charges on. That means its going to go at the center.
Decide which is the central atom in the structure. A step-by-step explanation of how to draw the CO3 2- Lewis Dot Structure Carbonate ionFor the CO3 2- structure use the periodic table to find the total nu. Carbon is the least electronegative.
Draw a skeleton structure in which the other atoms are single-bonded to the central atom. Draw a Lewis structure for CO2in which the central C atom obeys the octet rule and answer the following questions based on your drawing. That will normally be the least electronegative atom C.
Draw A Lewis Structure For Co2 lewis co2 dot structure dioxide carbon diagram electron pairs resonance electrons structures bonds elements many covalent lone valence oxygen ionic lewis co2 structure carbon dioxide draw centre oxygens either put going then side go dot diagram dioxide lewis molecular cross co2 structure structures silicon carbon draw chemistry co2 dot lewis diagram structure. That would mean that you would have a total of eight dots around the carbon thereby filling its octet. In order to complete the octets for all of the atoms in the structure you will need to form two double bonds.
These valence electrons are represented by drawing dots around the individual atoms hence the Lewis dot structure. To know the lewis structure of CO2 one should first understand what precisely the Lewis structure is. The number of valence electrons of carbon is 4.
The carbon dioxide chemical formula is CO2. Lewis dot structure is a pictorial representation of the arrangement of the valence shell electrons in the molecule. In order for each atom to achieve their respective octets they must form.
Carbon C is the least electronegative atom in the CO2 Lewis structure and therefore should be placed at the center of the structure. To get the valence electrons of carbonwe need to look at the electronic configuration of carbon. Drawing lines represent the bonds formed in the.
Drawing CO2 Lewis Structure is very easy to by using the following method. So lets multiply that together there. Here in this post we described step by.
On the periodic table Carbon is in group 4 or 14 sometimes. To draw the Lewis Dot structure for CO2 we have to find out the CO2 valence electrons firstWe express valence electrons as dots in CO2 lewis dot structure. And then Oxygen is in group 6 or 16.
This is the Lewis Dot Structure for CO2. You follow a sequence of steps. But we have two of them.
The octets of both of the oxygen atoms are also satisfied since the oxygens have a total of eight electrons around them thereby filling the valence shell. Carbon C is the least electronegative atom in the CO2 Lewis structure and therefore should be placed at the center of the structureThe Lewis structure for CO2 has a total of 16 valence electrons. Draw the Lewis dot structure of CO2 molecule.
The number of valence electrons of oxygen is 6. Draw The Lewis Structure Of Carbonate Ion CO2- 3. Lets draw the structure.
Hybrid Atomic Orbitals Chemistry LibreTexts Hybrid Atomic Orbitals Chemistry for Majors. C 61s²2s²2p² The highest value of principal quantum number here is n2. So well put the Carbon right here and.
Draw A Lewis Structure For Co2 How Many P Bonds Are There In A Co2 Molecule Molecular Structure and Polarity Chemistry 6 1 Lewis Electron Dot Diagrams Introductory Chemistry 5 2. Draw a trial structure by putting electron pairs around every atom until each. In order to complete the octets for all of the atoms in the structure you will need to form two double bonds.
The Lewis structure for CO2 has a total of 16 valence electrons. Were going to do the Lewis structure for CO2 Carbon dioxide.
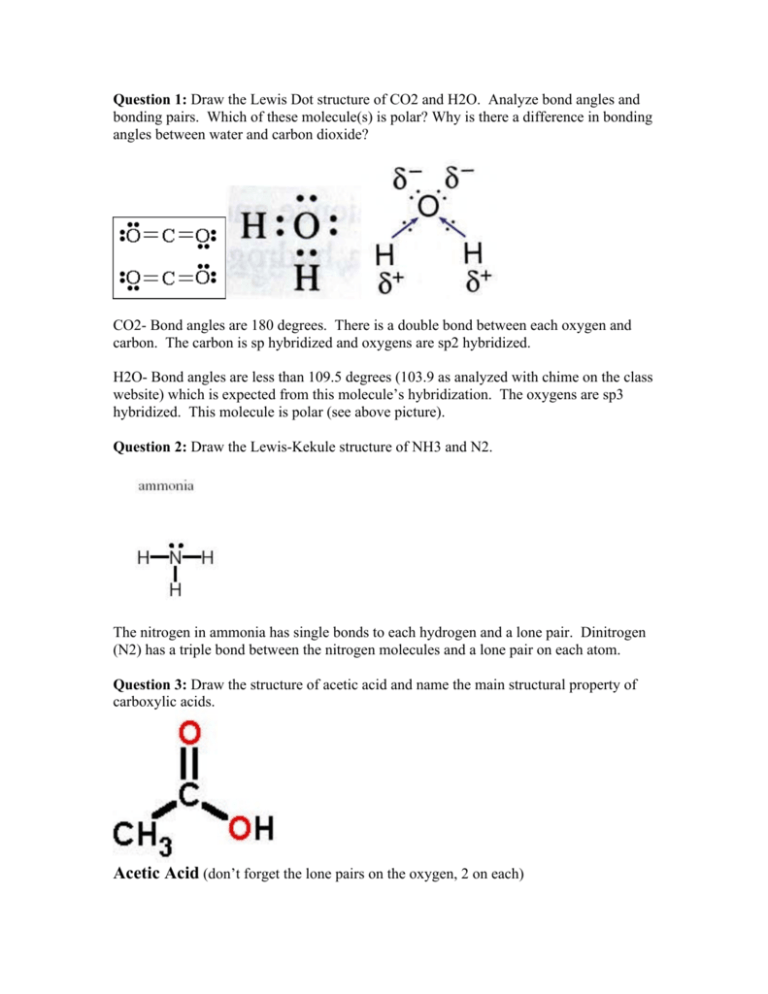
Question 1 Draw The Lewis Dot Structure Of Co2 And H2o Analyze

Co2 Lewis Structure Carbon Dioxide Youtube
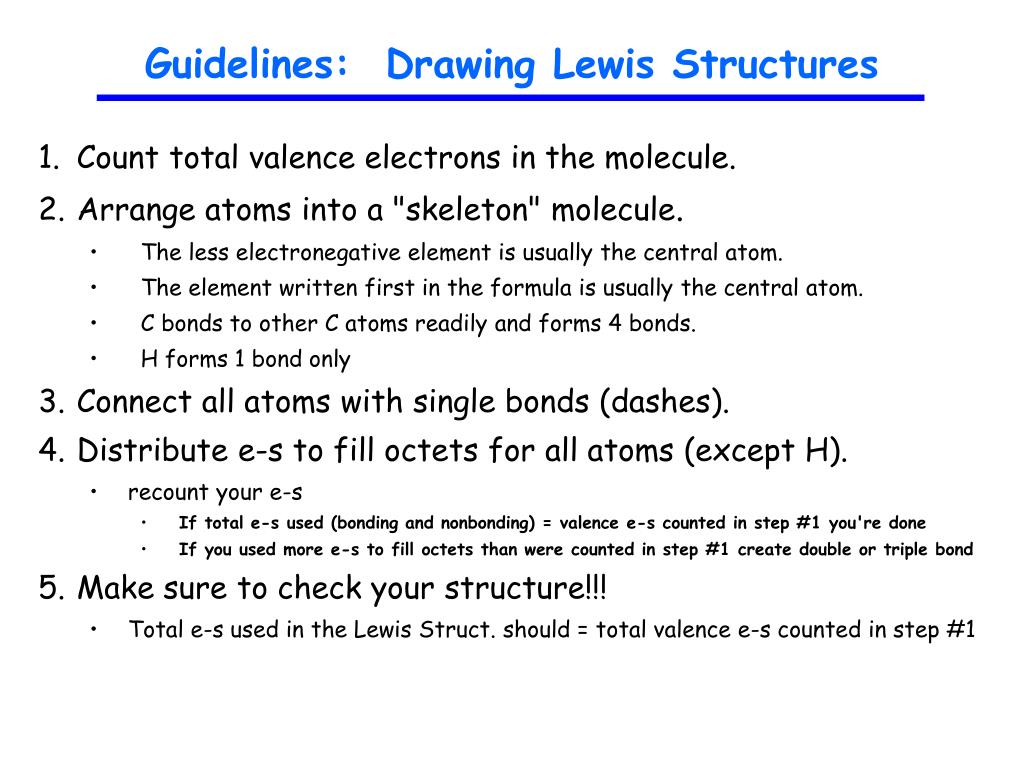
Ppt Guidelines Drawing Lewis Structures Powerpoint Presentation Free Download Id 1812949

Carbon Dioxide Lewis Structure How To Draw The Lewis Structure For Carbon Dioxide Youtube

Co2 Lewis Structure How To Draw The Dot Structure For Carbon Dioxide Youtube

Makethebrainhappy The Lewis Dot Structure For Co2
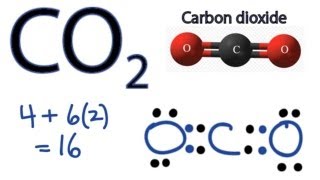
Co2 Lewis Structure How To Draw The Dot Structure For Carbon Dioxide Youtube

Co32 Lewis Structure How To Draw The Lewis Structure For Co3 2 Carbonate Ion Youtube

Lewis Structure For Co Carbon Monoxide Youtube
![]()
A Draw Lewis Structures For Co2 So2 And No3 B Give The Electron Pair Geometry And The Molecular Geometry Of The Three Species From Part A According To Vsepr C Are Co2
Co2 Lewis Structure Molecular Geometry And Hybridization
![]()
A Draw Lewis Structures For Co2 So2 And No3 B Give The Electron Pair Geometry And The Molecular Geometry Of The Three Species From Part A According To Vsepr C Are Co2

How To Draw The Lewis Structure Of Co3 2 Carbonate Ion Chemistry Youtube

Co2 Lewis Structure How To Draw The Dot Structure For Carbon Dioxide Youtube

Co2 Lewis Structure Carbon Dioxide Youtube

Co2 Lewis Structure Easy Hard Science
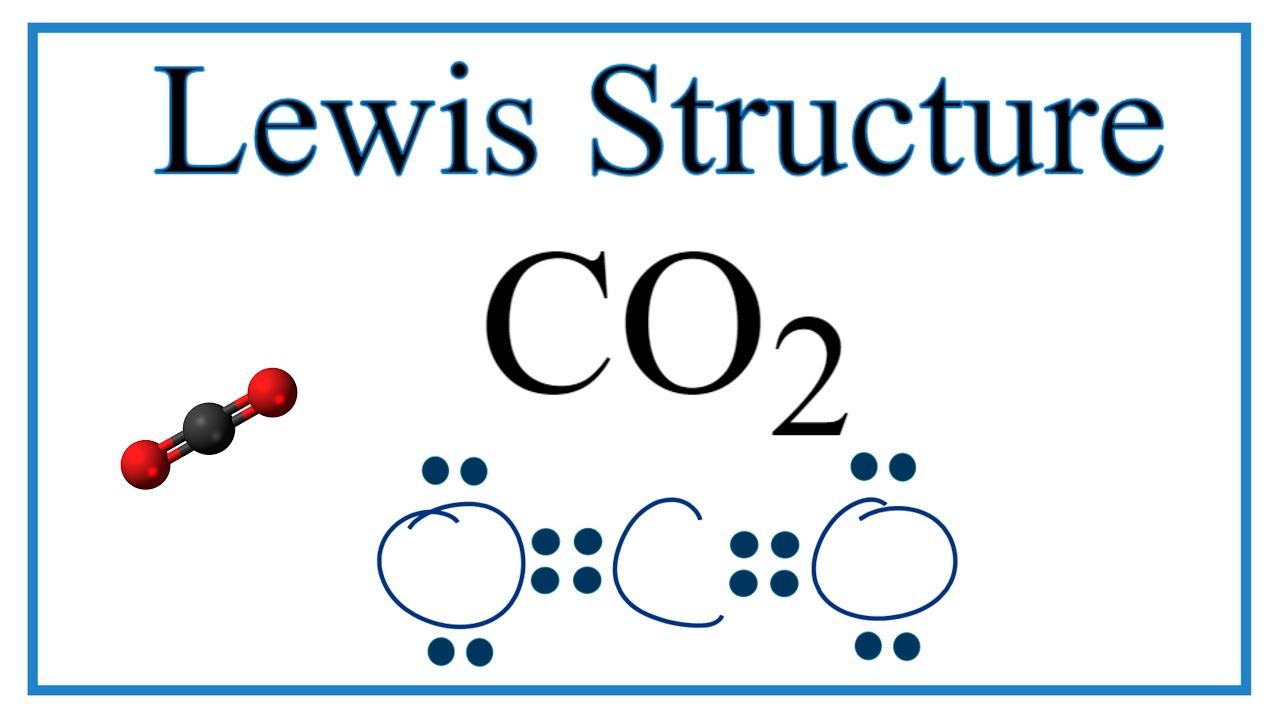
How To Draw The Lewis Dot Structure For Co2 Carbon Dioxide Youtube
Co2 Carbon Dioxide Lewis Structure And Shape