Draw The Lewis Structure For N2h2. What Is The Hybridization Of The N Atoms
The two atomic orbitals AOs involved in the formation of asigma bond between two hydrogen atoms in the molecule H2are. N2h2 lewis structure molecular geometry hybridization and mo diagram dinitrogen dihydride has the chemical formula of n2h2.
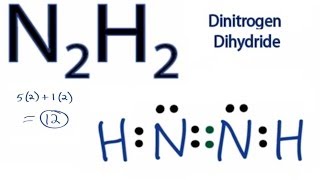
N2h2 Lewis Structure How To Draw The Dot Structure For N2h4 Chemical Bonding
Draw lewis structure for trans N2H2 What hybrid orbitals are involved in the sigma bonding between the two nitrogen atoms.

Draw the lewis structure for n2h2. what is the hybridization of the n atoms. Identify the hybridization of the N atoms in N2H4. The bond angle is 19 o 28. A step-by-step explanation of how to draw the N2 Lewis Dot Structure Nitrogen Gas - Diatomic NitrogenFor the N2 structure use the periodic table to find t.
The Lewis structure of N2H2 shows single bonds between the nitrogen and hydrogen atoms in the compound while the N atoms are connected via a double. This molecule is tetrahedral in structure as well as in shape since there are no lone pairs and the number of σ-bonds is equal to the steric number. It also exists in polymeric form as BeH2 n.
N2H2 is a chemical formula for a Diazene molecule which is also known as Nitrogen Hydride. Polar molecules interact through dipoledipole intermolecular forces and hydrogen bonds. The lewis structure for n2h2 hhnh shows1.
In the N 2 H 2 Lewis structure the two Nitrogen N atoms go in the center Hydrogen always goes on the outside. The molecule is made up of two hydrogen atoms and two nitrogen atoms. Osp sp3 O sp3d sp3 Osp2 Submit Request Answer Identify the hybridization of carbon atoms numbered 1-6 in the structure below.
Write the Lewis structure. Adding up the exponents you get 4. BeH2 Lewis Structure Molecular Geometry Hybridization and Polarity.
A Draw Lewisstructures for both molecules. The exponents on the subshells should add up to the number of bonds and lone pairs. σ bonds are stronger and result from end-to-end overlap and all single bonds are σ bonds.
It appears as an amorphous white solid at standard temperature and pressure. N 2 H 2 is straightforward with no double or triple bonds. It is the conjugate acid of a diazenide.
Not the number of bonds. Once you know which atom you count the number of atoms directly bonded to it NOTE. Calculate the total number of valence electrons present.
Hydrogen H only needs two valence electrons to have a full outer shell. In the n 2 h 2 lewis structure the two nitrogen n atoms go in the center hydrogen always goes on the outside. The valency of nitrogen is 3.
Each nitrogen has 4 electron pairs. Draw the Lewis Structure for the molecule to determine hybridization. Both types of bonds result from overlap of atomic orbitals on adjacent atoms and contain a maximum of two electrons.
There are three types of bonds present in the lewis structure of N2H4 one N-N and two H-N-H. To do that we need to do these steps. N2H2 Lewis structure Molecular Geometry Hybridization Bond Angle and Shape.
Explain how σ and π bonds are similar and how they are different. We are being asked to determine the hybridization around Nitrogen in N 2 H 2. Part A Draw the Lewis structure for N2H2.
It is an inorganic compound and comes under the category of alkaline earth hydride. Include all lone pairs of electrons. So for N2 each N has one lone pair and one triple bond with the other nitrogen atom which means it would be sp.
Whatis the hybrid state of each nitrogen and what nitrogen orbital isinvolved in forming the double bond. The number of atoms and the number of unshared pairs on the atom in the most stable Lewis structure. Molecular geometry Hybridization of the given molecule H2S is.
From what you have learned about molecular geometry after you draw N 2 Lewis structure you determine the arrangement of this molecule is linear also if there are only 2 atoms they are obviously gonna be and can only be linear dont you agree. Since iodine has a total of 5 bonds and 1 lone pair the hybridization is sp3d2. The fluorine and oxygen atoms are bonded to the nitrogen atom.
A linear shape only requires 2 orbitals. Add those two numbers together. First we will have to draw the Lewis Structure of N 2 H 2.
Table 32 on page 109 in the textbook is a great. The hybrid state ofthe central oxygen atom is. What is the hybridization on the N atoms.
Draw the electron dot structure of ozone. Fluorine has 1 bond and 3 lone pairs giving a total of 4 making the hybridization. Lewis structure of N2H4 is made up of two nitrogen and four hydrogens having two lone pairs on the nitrogen atomsone lone pair on each nitrogen and contain a total of 10 shared electrons.
Physics Draw the Lewis structure for N2H2 a neutral molecule 9752 results Science Draw the Lewis structure for N2H2 a neutral molecule. HCH2 367 Carbons 4 and 5 are sp3 carbons. Determine the central atom in this molecule.
DETERMINING THE HYBRIDIZATION OF NITROGEN IN AMMONIA NH 3 STEP-1. 2 hybridization is sp geometry linear. Therefore it forms 3 bonds with three hydrogen atoms.
In the Lewis structure for N 2 H 2. Draw the Lewis dot structure of N2H2. π bonds between the same.
Sp2 orbital on one N overlap with sp2 orbital on the other N to form the sigma bond. BeH2 is known as beryllium hydride or beryllium dihydride.
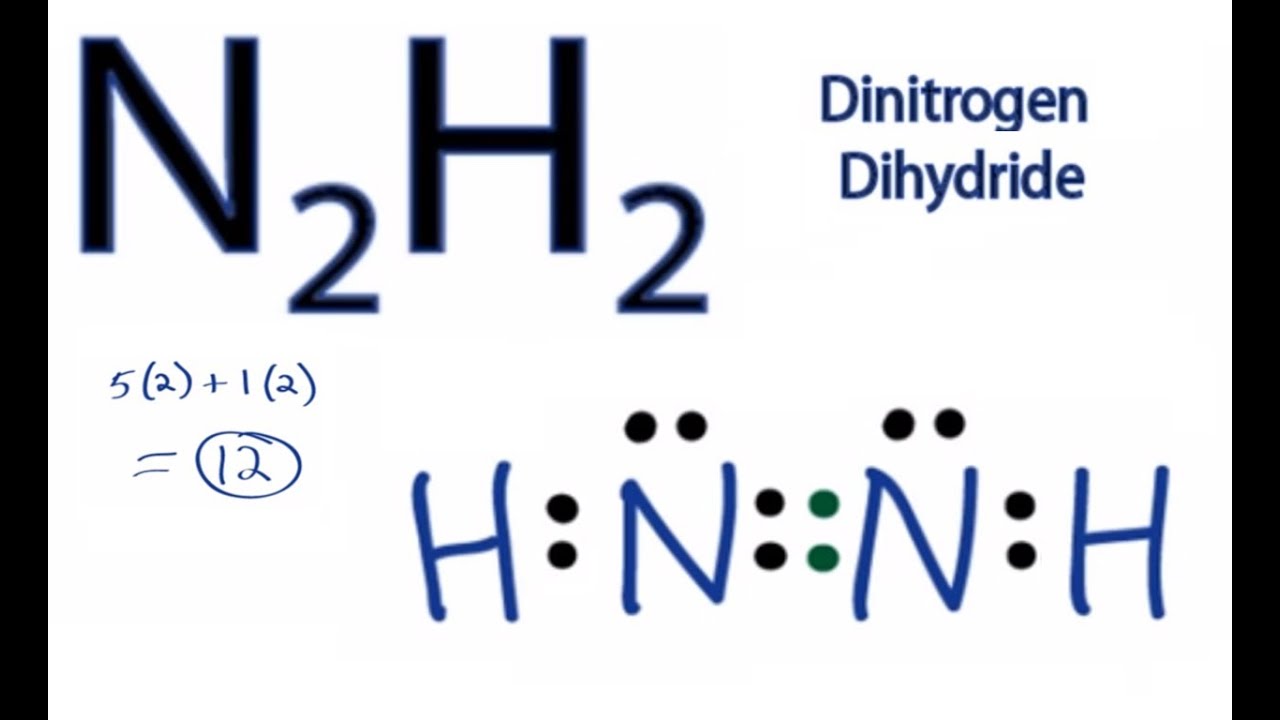
N2h2 Lewis Structure How To Draw The Dot Structure For N2h4 Chemical Bonding

Nh2nh2 Lewis Structure How To Draw The Lewis Structure For Hydrazine Youtube

N2h2 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

Part A Draw The Lewis Structure For N2h2 What Is The Chegg Com

Determine The Hybridization Around Nitroge Clutch Prep
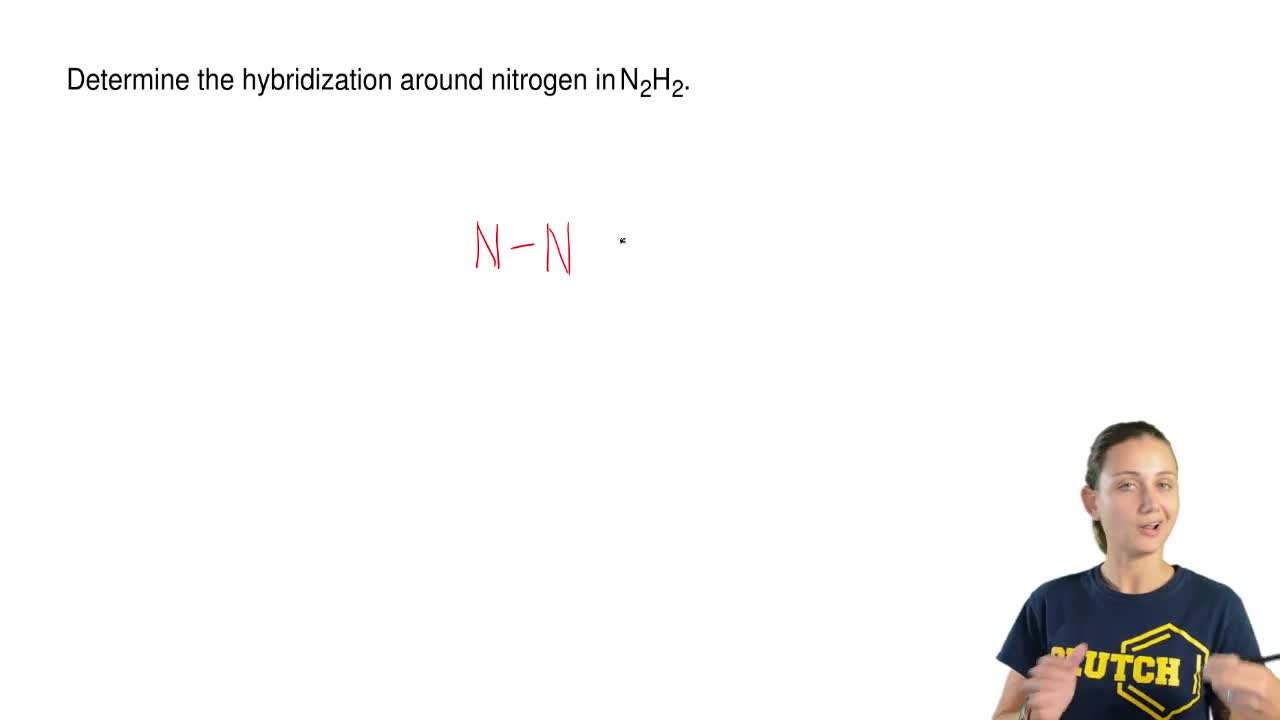
Determine The Hybridization Around Nitroge Clutch Prep
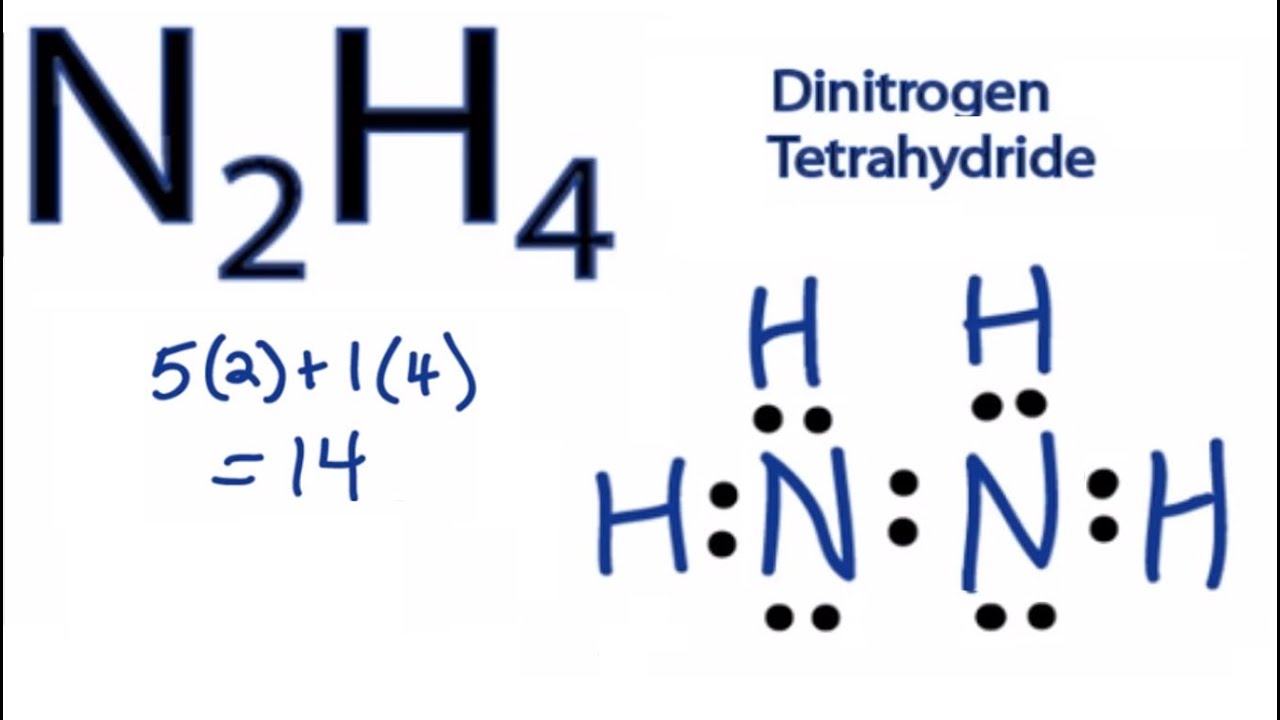
N2h4 Lewis Structure How To Draw The Lewis Structure For N2h4 Youtube

N2h2 Lewis Structure Molecular Geometry Hybridization Bond Angle And Shape
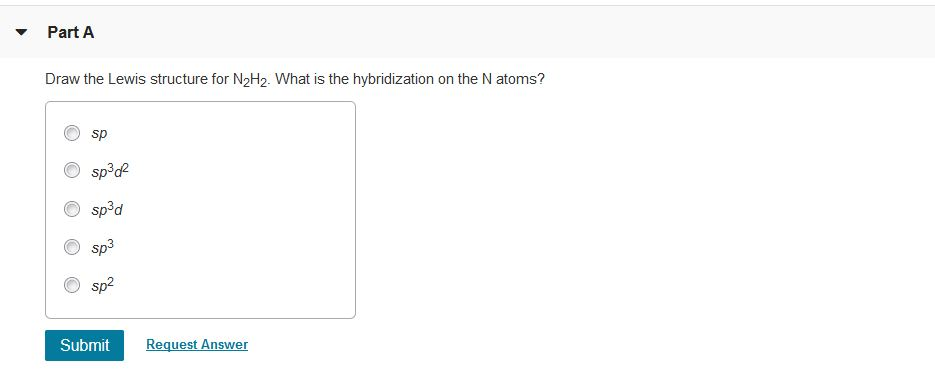
Part A Draw The Lewis Structure For N2h2 What Is The Chegg Com

N2h2 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist
Formal Charge Problems 4 N3 Youtube

N2h2 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

Draw The Molecules Include All Lone Pairs Of Electrons N2h2 N2h4 C2h2 C2h4 H3coch3 Study Com
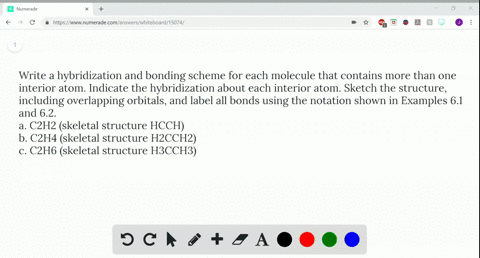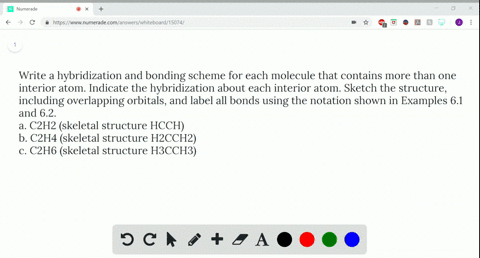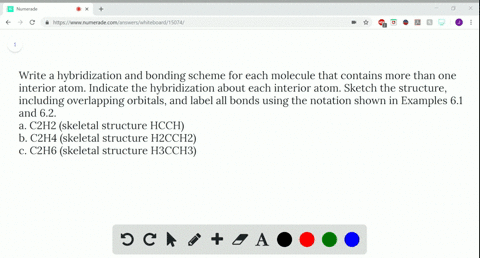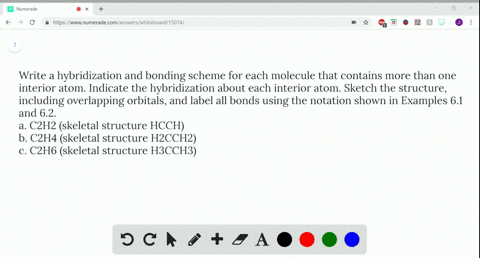
Solved Write A Hybridization And Bonding Scheme F

N2h2 Molecular Geometry Bond Angles And Electron Geometry Youtube

Draw Lewis Structures For The Following Mo Clutch Prep

N2h4 Molecular Geometry And Bond Angles Actual Bond Angle Is Less Than 109 5 Degrees Youtube
N2h2 Lewis Structure Molecular Geometry Hybridization Bond Angle And Shape

Determine The Hybridization Around Nitroge Clutch Prep