Draw The Lewis Structure Of N2h4
Lewis structure of N 2 H 4. The drawing of the Hydrazine molecule looks correct with the proper angles for a pyramidal structure on both ends.
Also the arrows showing the lower electronegativity to the higher negativity are correct as the Nitrogen has a higher level than the Hydrogen.

Draw the lewis structure of n2h4. Count total valence electron in N2H4. Draw the molecule by placing atoms on the grid and connecting them with bonds. You have two Nitrogens.
Find the number of nonbonding lone pairs e-. Find octet e- for each atom and add them together. So in the first step we have to count how many valence electrons available for drawing the Lewis structure of N2H4.
Lewis Dot Structure of CH3COOH Acetic Acid YouTube. Include all lone pairs of electrons and hydrogen atomsView Available Hint s Namio9408 is waiting for your help. A step-by-step explanation of how to write the Lewis Dot Structure for NH4 Ammonium IonFor the NH4 Lewis structure calculate the total number of valenc.
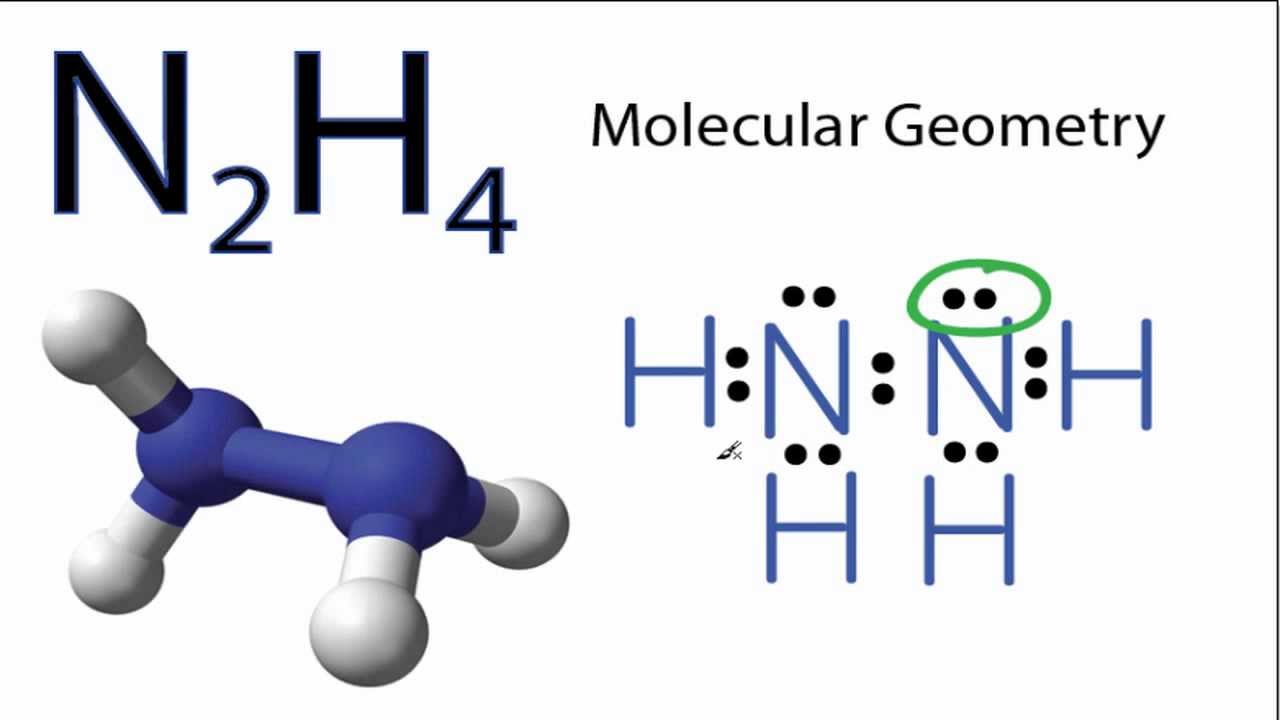
Back in the center twelve and fourteen. Drawing the Lewis Structure for N 2 H 4. Lewis structure for N 2 H 4 is as shown in the figure.
The two atomic orbitals aos involved in the formation of a sigma bond between two hydrogen atoms in the molecule h2 are draw. N 5A 2 5 e 10 e H 1A 4 1 e 4 e Total. As we know lewiss structure is a representation of the valence electron in a molecule.
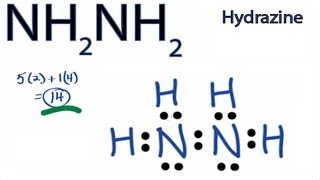
A stepbystep explanation of how to draw the n2h4 lewis structure dinitrogen tetrahydride or hydrizine. 14 valence e. Draw the Lewis structure of N2H4 whose skeletal structure is H2NNH2.
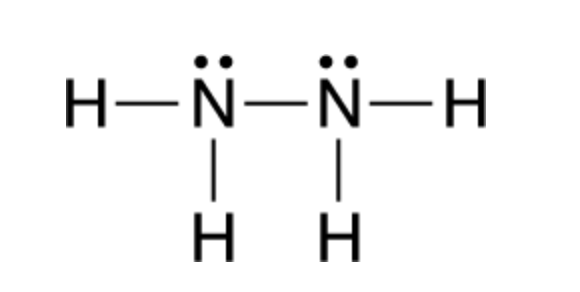
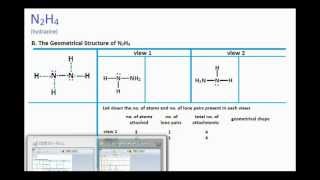
Trigonal pyramidal and trigonal planar and and. Include all hydrogen atoms and nonbonding electrons. -The geometry of N2H4 structure but there is one lone pair making the molecular geometry about each interior atom n2h2 molecular geometry Draw.
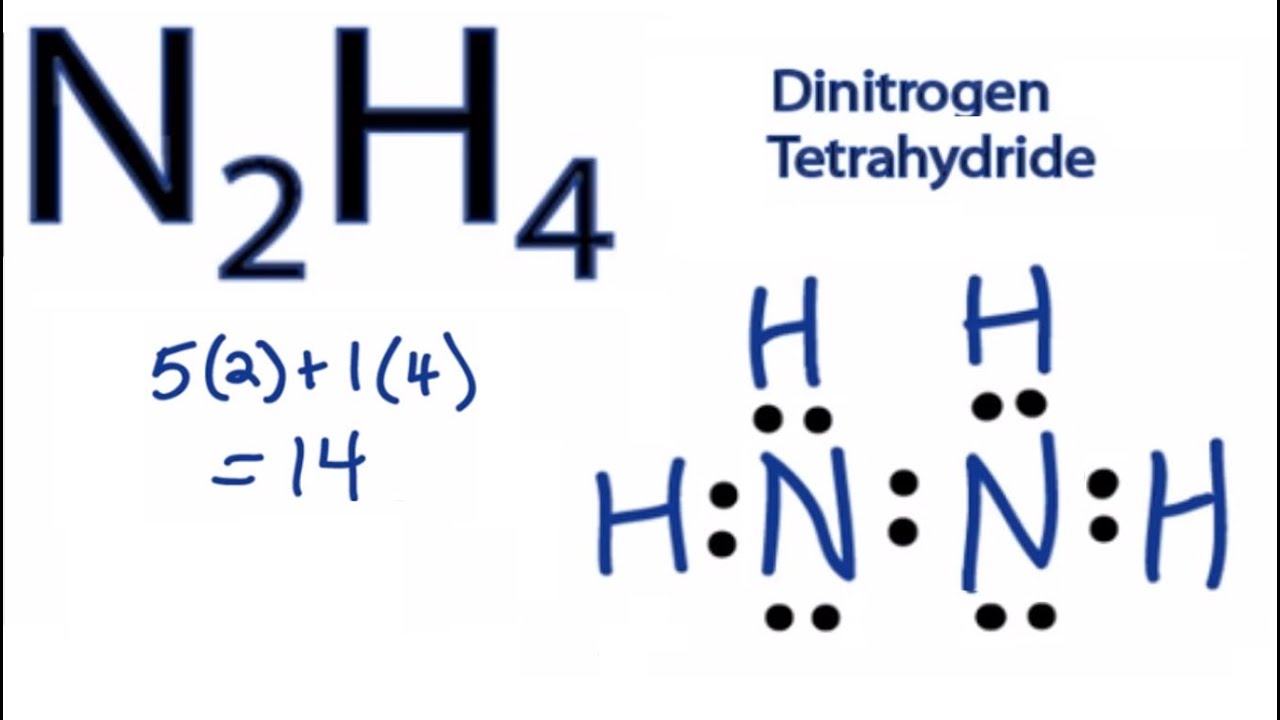
In the N2H4 Lewis structure the two Nitrogen N atoms go in the center Hydrogen always goes on the outside. Follow some steps for drawing the lewis dot structure of N2H4. Hydrogen H only needs two valence electrons to have a full outer shell.
Chemistry learning made easy. Lets do the N2H4 Lewis structure. Draw Lewis structures for the following molecules Be sure.
Well determine the n2h4 molecular geometry with respect to nitrogen on right the other atom will have same shape since they are symmet. In the Lewis structure for N2H4 there are a. Draw The Lewis Structure For The Rocket Fuel Hydra.
Subtract step 3 number from step 1. N2H4 is straightforward with no double or triple bonds. Subtract step 1 total from step 2This step gives you number of bonding e-.
Add your answer and earn points. Now we are going to learn how to draw this lewis structure. Hydrogen H only needs two valence electrons to have a full outer shell.
In the Lewis structure for N2H4 there are a total of 14 valence electrons. You showed all three forces of attraction which take place between N2H4 and another. Albr3 Lewis Structure How to Draw the Lewis Dot Structure.
Use information from step 4 and 5 to draw the lewis structure. In the N2H4 Lewis structure the two Nitrogen N atoms go in the center Hydrogen always goes on the ou Weve used six eight ten. We first need to determine the total number of valence electrons present in the molecule.
Draw the lewis dot structure of n2h2. Leaves two possible configurations. Were being asked to draw a Lewis structure for N 2 H 4.
For structure calculate total number valence elect. Nitrogen has five valence electrons. Hydrogen H only needs two valence electrons to have a full outer shell.
The octet rule provides a guide to drawing the Lewis structures of compounds. Draw the Lewis structures of N2H4 N2H2 and N2Draw the molecules by placing atoms on the grid and connecting them with bonds. In the N2H4 Lewis structure the two Nitrogen N atoms go in the center Hydrogen always goes on the outside.
N 2 H 4 is straightforward with no double or triple bonds. In the N 2 H 4 Lewis structure the two Nitrogen N atoms go in the center Hydrogen always goes on the outside. Trigonal pyramidal having 3 electron pairs.
Draw The Lewis Structure For N2h4.

Hydrazine N2h4 Lewis Structure
N2h4 Molecular Geometry And Bond Angles Actual Bond Angle Is Less Than 109 5 Degrees Youtube
Answer The Following Questions About N2 And N2h4 Chegg Com

Hydrazine N2h4 And Carbon Disulfide Cs2 Clutch Prep

Draw The Lewis Structure For N2h4 Clutch Prep
N2h4 Lewis Structure How To Draw The Lewis Structure For N2h4 Youtube
What Is The Lewis Dot Structure For N2h4 How Is It Made Quora

What Is The Lewis Structure For N2h4 Study Com
What Is The Lewis Dot Structure For N2h4 How Is It Made Quora
Why Does N N Dimethylhydrazine Have Hydrazine When It Has N2h2 Not N2h4 Hydrazine Why Isn T It Called Dimethyldiimide Quora

Draw The Lewis Structure For N2h4 Clutch Prep

The Lewis Structure Of Hydrazine Youtube
Nh2nh2 Lewis Structure How To Draw The Lewis Structure For Hydrazine Youtube
N2h4 Lewis Structure And Molecular Geometry Youtube
What Is The Lewis Dot Structure For N2h4 How Is It Made Quora
What Is The Lewis Dot Structure For N2h4 How Is It Made Quora
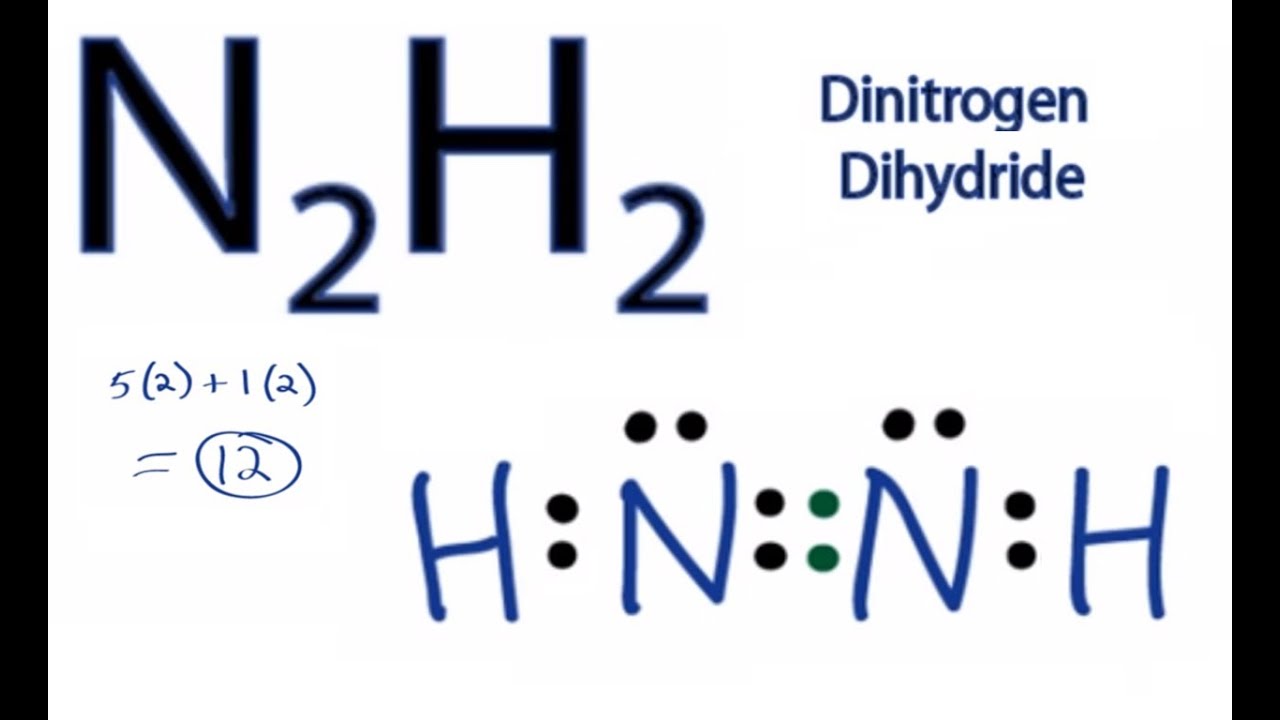
N2h2 Lewis Structure How To Draw The Dot Structure For N2h4 Chemical Bonding

Draw The Molecules Include All Lone Pairs Of Electrons N2h2 N2h4 C2h2 C2h4 H3coch3 Study Com

Part D Draw The Lewis Structures Of N2h4 Clutch Prep