Draw Lewis Structure Pbr3
Br group 7 has 7 valence electrons but since it has 3 atoms of Br it will be 73 21 valence electrons. Complete the structure by adding bonds and nonbonding electrons.

Pbr3 Lewis Structure Molecular Geometry Polarity And Hybridization Techiescientist
P-Br We have found out that PBr3 is polar due to asymmetrical property and has a trigonal bipyramidal shape.

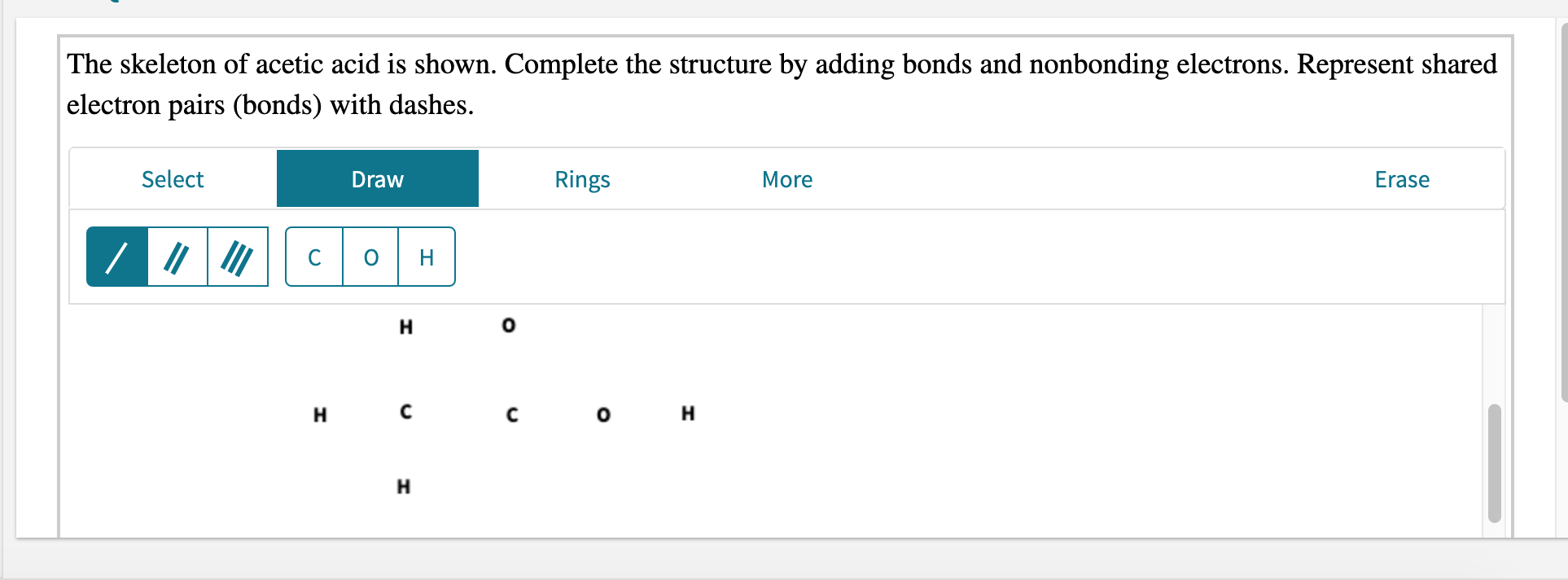
Draw lewis structure pbr3. The skeleton of acetic acid is shown. Draw the Lewis structure of NBr3. Include all the lone pairs.
Determine the electron geometry and molecular shape of this molecule. Draw the Lewis dot structure for PBr3. Here in this post we described step by step method to construct CH3I Lewis Structure.
If we calculate the number now 37526. Experts are tested by Chegg as specialists in their subject area. Single bonds double bonds The Lewis dot structure for PBR3 has triple bonds and a TOTAL of lone pairs.
There has been one report of PBr3 effectiveness on real-scale flames indicating an effectiveness two orders of. What is the hybridization of the central atom in the ion. Draw a Lewis dot structure for PBr3.
By signing up youll get thousands of step-by-step solutions to your. Youve used 6 of the 26 valence electrons in single bonds. If a given feature is not applicable enter 0.
Put P in the middle. Boron B doesnt need 8 valence electrons to have an octet Boron often only needs 6. Drawing the Lewis Structure for BBr 3.
Show transcribed image text. What is the formal charge bonding or non bonding. Phosphorus halides including phosphorus trichloride and phosphorus tribromide have been considered as potential replacements for Halon 1301 as fire suppressants.
It has sp3 Hybridization and the bond angle is approximately 1095. We have found out the most appropriate Lewis Structure where we checked the single bonds formed between P and each Br atom. So PBr3 the first thing we do as always is count the valence electrons.
In this case Br 7 and P 5. Drawing PBr3 Lewis Structure is very easy to by using the following method. Okay now we have the number of valence electrons.
Distribute the 20 as lone pairs and your structure. You have 20 remaining. Three pairs will be used in the chemical bonds between the P and Br.
In the Lewis structure for PBr 3 there are a total of 26 valence electrons. A step-by-step explanation of how to draw the Br3 - Lewis Dot Structure Tribromide ionFor the Br3 - structure use the periodic table to find the total num. Draw the Lewis structure for PBr3 and label the formal charges.
If you can do those Lewis structures PBr 3 will be easy. The phosphorus tribromide chemical formula is PBr3. This makes 20 which is how many you have remaining.
See the answer. Coming to electronic configurations For phosphorus P1s2 2s2 2p6 3s2 3p3. See the answer See the answer done loading.
P group 5 has 5 valence electrons and is the central atom since all other atoms are bonded to it. A Nonpolar B Polar. Drawing CH3I Lewis Structure is very easy to by using the following method.
The carbon iodine and hydrogen elements come as the member of the carbon halogen and hydrogen family groups from the periodic table respectively. Represent shared electron pairs bonds with dashes. PBr 3 is similar to PCl 3 and PF 3.
Do NOT leave it blank. Each Br needs 6 while the P needs 2 more to become stable. The next step is a sketch it doesnt matter how we draw or anything.
Then describe it by filling in the blanks in the following statement with numbers. Here in this post we. To determine the Lewis structure of PBr3 we first count the total number of valence electrons of each element.
Who are the experts. In the Lewis structure of PBr3 there are three bonding pairs of electrons and one lone pair of electrons on the central atom. The BBr 3 Lewis structure is similar to BF 3 and BCl 3 since F and Cl are in Group 7 and have 7 valence electrons.
Attach the three bromines. Is this molecule polar or. Draw the Lewis structure of PBr3 and then determine if the molecule is polar or nonpolar.
Draw the Lewis Structure for phosphorus tribromide Pbr3. Drawing the Lewis Structure for PBr 3. The molecule is trigonal pyramidal-shaped and is a polar molecule.
Which is its electron geometry. Draw the Lewis structure of PBr3. There are a total of 26 valence electrons for PBr3.
In the PBr 3 Lewis structure Phosphorus P is the least electronegative so it goes in the center. We review their content and use your feedback to keep the quality high. Which is its molecular geometry.
This homework is due tomorrow by 8am.

Pbr3 Lewis Structure Molecular Geometry Polarity And Hybridization Techiescientist

Pbr3 Lewis Structure Lewis Structure Of Pbr3 Phosphorus Tribromide Draw Lewis Structure For Pbr3 Youtube

Draw The Lewis Structure For Phosphorus Tribromide Pbr3 1 Clutch Prep

6 2 Lewis Structures Introductory Chemistry
Is Pbr3 Polar Or Non Polar Quora
Draw The Lewis Structure Of Pbr3 Include All Chegg Com

6 2 Lewis Structures Introductory Chemistry

What Is The Molecular Geometry Of Pbr3 Enter The Molecular Clutch Prep
Pbr3 Lewis Structure Molecular Geometry Hybridization And Polarity
Diagram Lewis Dot Diagram For Pbr3 Full Version Hd Quality For Pbr3 Snadiagram Bmwe21fansclub It

Pin By Sarah Hawkins On Chemistry Class Molecular Geometry Teaching Chemistry Chemistry Class

Pbr3 Lewis Structure How To Draw The Lewis Structure For Pbr3 Youtube

Pbr3 Molecular Geometry Shape And Bond Angles Youtube

Pbr3 Lewis Structure How To Draw The Lewis Structure For Pbr3 Youtube

Pbr3 Lewis Structure Molecular Geometry Polarity And Hybridization Techiescientist

6 2 Lewis Structures Introductory Chemistry

What Is The Lewis Structure Of Pbr3 How Is It Determined Quora

What Is The Lewis Structure For Pbr3 Study Com

What Is The Molecular Geometry Of Pbr3 Enter The Molecular Clutch Prep