How Many Valence Electrons In Clo3-
Also note that you should put the ClO- Lewis structure in brackets with a negative sign on the outside to. It has 17 electrons distributed in 3 energy shells.

Science Coverage Is Hf Polar Or Nonpolar Electron Affinity Covalent Bonding Molecular Geometry
The combination of two lithium atoms to form a lithium molecule Li2 is analogous to the formation of H2 but the atomic orbitals involved are the valence 2s orbitals.

How many valence electrons in clo3-. As a result they will be pushed apart giving the ClO3- molecule a trigonal pyramidal geometry or shape. OH- Oxygen has 6 valence electrons Hydrogen has 1 valence electron Need 1 more to get the charge of -1 Total number 8 Example 2. In next steps we are going to mark those 16 lone pairs on oxygen atoms and chlorine atoms as bonds and lone pairs.
Asked Jan 10 2019 in Chemistry by Steve. There are a total of 26 valence electrons for ClO 3-. Valence electrons of chlorine.
The boron atom donates its valence electrons to the chlorine atom and the chlorine atom receives those electrons. Valence electrons Total number of electrons in outermost shell also called as valence shell is called as valence electrons. Secondly how many lone pairs are in ClO2.
Here 9 pi-bonds and 9 sigma bonds are present. Answered Jan 10 2019 by Johanny. Center atom of ClO3-ion To be the center atom ability of having greater valance is important.
What is the calculation for the number of valence electrons in ClO3-. In order to calculate the formal charges for ClO3- well use the equationFormal charge of valence electrons nonbonding val electrons bonding e. D 0 votes.
There also exists a negative ionic charge of -1 in the ion. To determine the number of electrons in polyatomic ions you will need to determine the number of valence electron for each element present in the ion and make a correction given the charge of the ion. The total number of electrons present in the valence shell of an atom are called valence electrons and there are a.
In doing so the electron pair geometry of the molecule is tetrahedral and the molecular geometry is bent. See the Big List of Lewis Structures. Worked like a charm.
NH_4 Nitrogen has 5 valence electrons Hydrogen has 1 valence electron x 4 because you have 4 atoms of H Need to remove 1 electron. Also how many bonds are present in CN. And a double bond contains a single bond and a pi bond.
Once we know how many valence electrons there are in ClO- we can distribute them around the central atom with the goal of filling the outer shells of each atom. Two inner shell core electrons in the 1s orbital and four valence outer most shell electrons in the 2s and 2p orbitals. How many valence electrons are present in the Lewis dot formula of the chlorate ion ClO3-.
Remember to put brackets around the Lewis structure along with a negative sign to show that it is an ion. The valence electrons on chlorine are 7. To know the bond formation and the arrangement lets go through the valence electrons of all the atoms in the molecule.
Therefore this ion has seven valence electrons from chlorine and 18 valence electrons from the three oxygen atoms which means it has 25 valence electrons overall. Chlorine can show valence 7. In ClO3- the central atom is chlorine and its valency is 6 because there are three double bonds between chlorine and oxygen.
For ClO 4 - there are 32 valence electrons so total pairs of electrons are 16. E 3 N-Cl bonds and 9 lone pairs of electrons. An electron in an atoms valence shell.
Each of the two lithium atoms has one valence electron. The atomic number of chlorine is 17. Since there are two lone pairs on chlorine the electron pair repulsion will result in a bond.
Predict The Total Number Of Valence Electrons In The Following. 14 valence electrons. Once we know how many valence electrons there are in ClO- we can distribute them around the central atom with the goal of filling the outer shells of each atom.
ClO3 Valence Electrons A Chlorate ion consists of one chlorine atom and three oxygen atoms. You should check the formal charges to make sure this is the best structure since the molecule has Chlorine as a central atom. As last shell has 3 electrons it has 3 valence electrons.
A triple bond contains 2 pi bond and a sigma bond. Answered Jun 26 2017 by Carissa. Now consider Chlorine with electronic configuration Cl287 here.
20 valence electrons. Hence we have two valence electrons available for the σ2s bonding molecular orbital. Atomic carbon has six electrons.
Thanks for your help. The atoms attached to chlorine are 3 and the charge is -1. The chlorite ion.
2 bonds per cyanide molecule and there are 4 cyanide molecule attached to double bonded carbon and 1 pi bond of ethylene. Click to read full detail here. The electronic configuration of nitrogen is 2 8 7.
What is the Lewis structure for ClO. Asked Jun 26 2017 in Chemistry by OneMoreTime. Total electron pairs are determined by dividing the number total valence electrons by two.
It is present in group 7 of the Periodic Table of Elements. ClO3-there are 26 valence electrons so total pairs of electrons are 13. A lone pair electron or an electron which is part of a covalent bond.
Adding to that how many valence electrons does C have. Being in group 7 of the periodic table Chlorine has seven valence electrons with a valency of -1. The electron configuration of chlorine shows that the valence electrons of chlorine are seven.
Answered Jan 10 2019 by ziziz. Furthermore what is the hybridization of CL in ClO 2.

126 Hso4 Lewis Structure How To Draw The Lewis Structure For The Bisulfate Ion Youtube Science Chemistry Chemistry Organic Chemistry
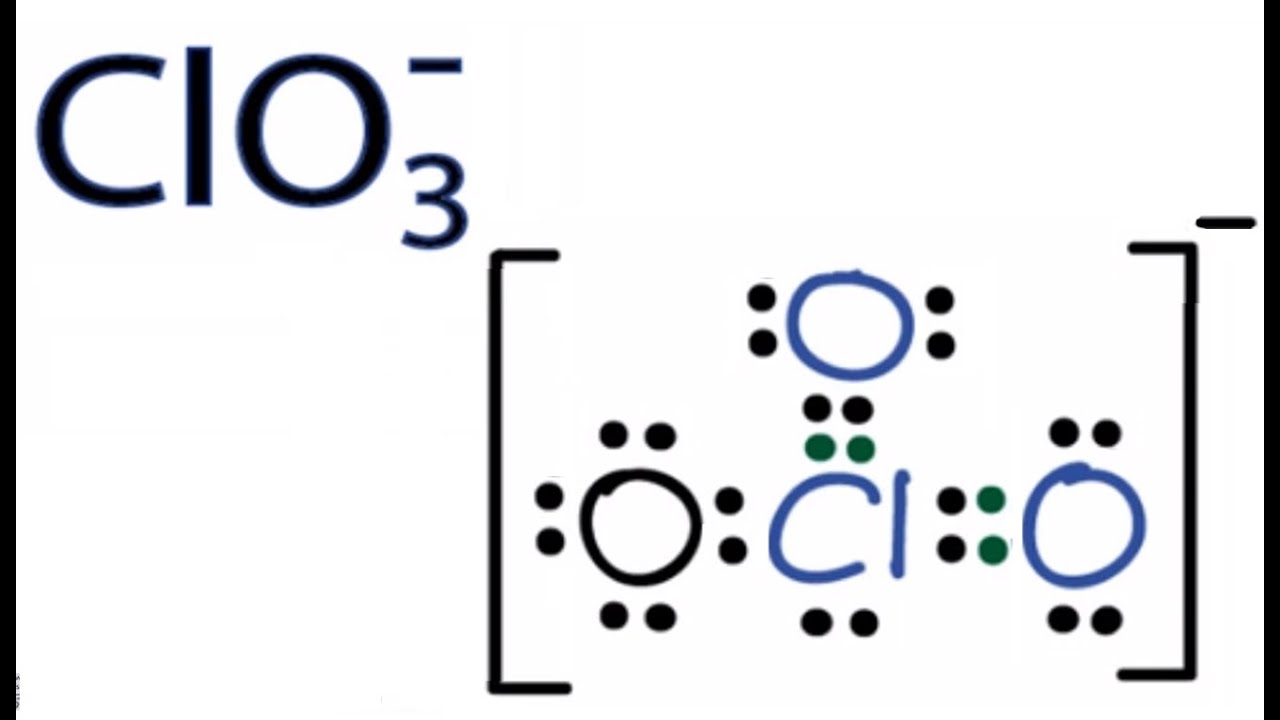
Clo3 Lewis Structure How To Draw The Lewis Structure For Clo3 Chlorate Ion Youtube

Clo3 Lewis Structure Molecular Geometry Hybridization And Polarity Techiescientist

Becl2 Lewis Structure Beryllium Chloride In 2021 Math Equations Lewis Molecules

Calculating No3 Formal Charges Calculating Formal Charges For No3 Chemistry Classroom Science Chemistry Chemistry

126 Clo3 Lewis Structure How To Draw The Lewis Structure For Clo3 Chlorate Ion Youtube Chemistry Classroom Science Chemistry Chemistry

Science Coverage Valency Of Lithium How Many Valence Electrons Do In 2021 Electron Configuration Electrons Chemistry

Cf2cl2 Lewis Structure How To Draw The Dot Structure For Cf2cl2 Dichl Drawings Dots Lewis
Clo3 Lewis Structure Molecular Geometry Hybridization Shape
Clo3 Lewis Structure Chlorate Ion Youtube

Science Coverage Is Clo3 Polar Or Nonpolar In 2021 Molecular Geometry Covalent Bonding Oxidation State

Pcl3 Lewis Structure Phosphorus Trichloride In 2021 Math Equations Lewis Chemical Formula
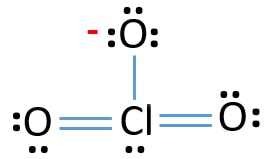
Lewis Structure Of Clo3 Chlorate Ion

Clo3 Lewis Structure Chlorate Ion Youtube

Science Coverage Valency Of Cesium How Many Valence Electrons Doe In 2021 Electron Configuration Electrons Noble Gas

Pcl3 Lewis Structure Phosphorus Trichloride In 2021 Math Equations Lewis Chemical Formula


