Is Sef4 Polar Or Nonpolar
Using our fun classroom quiz game Quizalize and. Learn to determine if CHF3 Trifluoromethane is polar or non-polar based on the Lewis Structure and the molecular geometry shapeWe start with the Lewis S.

Is Ch4 Methane Polar Or Nonpolar Youtube
SeF4 is a polar molecule because of asymmetrical geometry that causes the non-uniform distribution of charge in the molecule.

Is sef4 polar or nonpolar. Xenon tetrafluoride XeF4 is a non-polar chemical compound owing to its symmetrical. KrF4 squares non-polar bond dipoles are canceled. Again each molecule has the same number of atoms but a different structure due to the different number of lone pairs around the central.
Again each molecule has the same number of atoms but a different structure because of differing numbers of lone pairs around the central atom. Hence its geometry is trigonal bipyramidal and shape is see saw. Here there is only one lone pair around the central atom Sulfur which is an odd number.
Answer SOF4 Thionyl tetrafluoride is Polar What is polar and non-polar. Hence this molecule will be non polar. Bond dipoles do not cancel.
Ill tell you the polar or nonpolar list below. This makes the compound nonpolar. Polar In chemistry polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment.
KrF4 Square planar nonpolar. If there are some lone pairs of electrons around the central atom and if there is an odd number the molecule is polar. Hi Guys Today we are going to determine the polarity for the SeF4 molecule.
In the SeF4 lewis structure a total of 13 lone pairs and 4 bond pairs are present. The dipole moment is the major aspect for any compound to be polar or nonpolar. Polar NH3 C2H5OH CH3Cl and K2SO4 actually the last one is ionic but ions can be thought of as polar because they have oppositely charges Nonpolar Br2 SiF4 C3H8.
Polar In chemistry polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment. Due to its geometrical structure SeF4 is polar. It has the chemical name of Selenium Tetrafluoride and is used as a fluorinating.
Answer SeF4 is Polar What is polar and non-polar. Is SeF4 polar or nonpolar. The EN difference between hydrogen and sulfur is 04 so hydrogen and sulfur form non-polar bonds.
Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms. If you want to quickly find the word you want to search use Ctrl F then type the word you want to search. By Dec 2 2020 Uncategorized 0 comments Dec 2 2020 Uncategorized 0 comments.
Why is SeF4 polar. Yesselenium tetrafluoride that means SeF4 is a polar molecule because it is of AB4E type molecule. Is BH3 polar or nonpolar.
Second is SeF4 polar or non-polar. Hence its geometry is trigonal bipyramidal and shape is see saw. Polar In chemistry polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole or multipole moment.
So SF4 is polar. This means that SeF4 has a trigonal-bipyramidal structure with 4 bond pairs and 1 lone pair. Moreover Fluorine is more electronegative than Sulfur due to which the overall charge distribution of a molecule is uneven resulting in a polar molecule and give 0632 D dipole moment.
This is all about the article Is XeF4 Polar or Nonpolar. A polar molecule with two or more polar bonds must. Yesselenium tetrafluoride that means SeF4 is a polar molecule because it is of AB4E type molecule.
Oscillating saw the dipoles of the polar bond are uninterrupted. All the polar molecules have a net dipole moment and all nonpolar have zero dipole moment. SeF6 is not polar.
The geometrical structure is seesaw the two opposite F atoms would cancel each others charge however the other two which are about 120 angle degrees away from the lone pair electrons on Se would maintain their charge thus making SeF4 polar. XeF4 is a nonpolar molecule so that its dipole moment is 0D Zero. The bonding in CF4 will be covalent because of net dipole zero in this case.
Is sef4 polar or nonpolar. Question Is SeF4 polar or nonpolar. The molecular geometry of SeF4 is see-saw and electron geometry is trigonal bipyramidal.
SF4 Sulfur tetrafluoride is polar in nature as sulfur atom consists of a lone pair on it due to which the shape of the molecule becomes asymmetric ie. Just like this molecule SF4. As discussed the XeF4 molecule.
Polar molecules must contain polar bonds due to a difference in electronegativity between the bonded atoms. Sif4 polar or nonpolar. Most of the atoms have less than eight electrons in their valence shells except the noble gases in the group 18 of.
So is SF4 Polar or Nonpolar. A polar molecule with two or more polar bonds must. List molecules polar and non polar.
Is KrF4 polar or nonpolar.
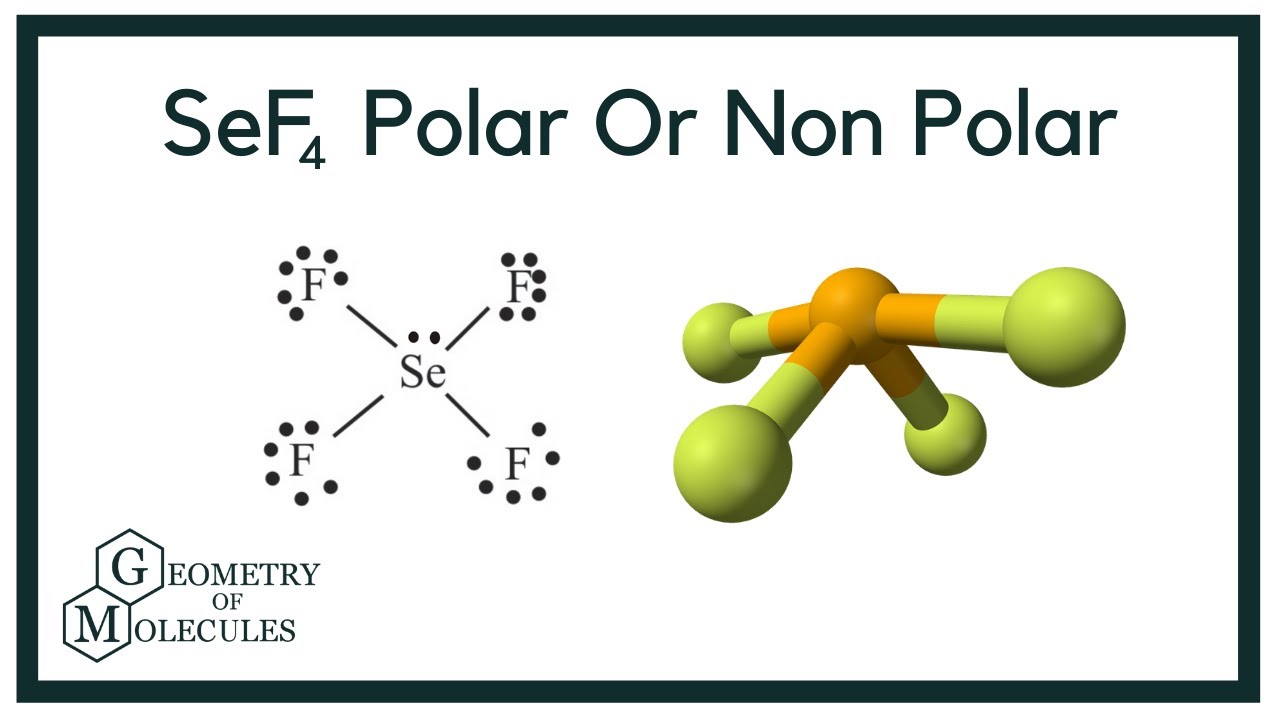
Is Sef4 Polar Or Non Polar Selenium Tetrafluoride Youtube
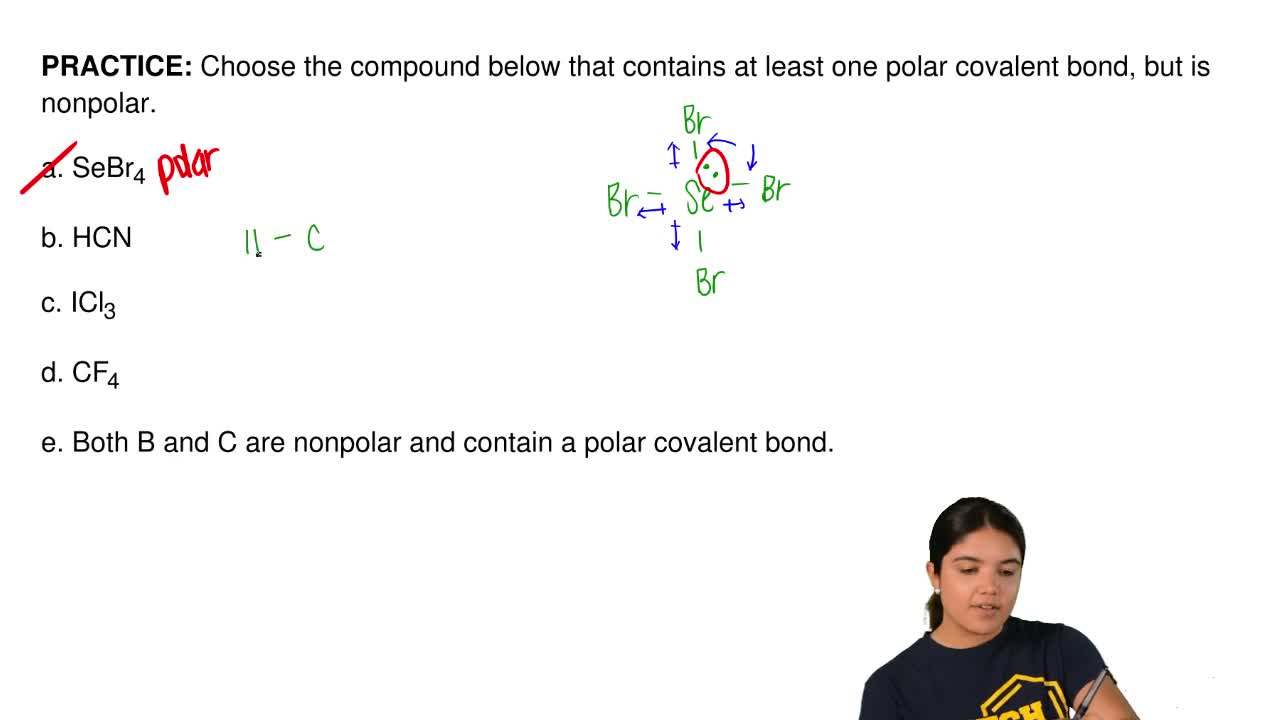
Choose The Compound Below That Contains At Least One
Is Co2 Polar Or Non Polar Quora

Is Of2 Polar Or Nonpolar Oxygen Difluoride In 2021 Oxygen Chemical Formula Molecules

Is Hbr Polar Or Nonpolar Hydrogen Bromide Youtube

Which Of The Following Statements About Sf5 Is True 1 The Ion Has Polar Bonds And

Is Cf4 Polar Or Non Polar Carbon Tetrafluoride Youtube
Polar And Nonpolar Molecules Is It Polar Or Nonpolar Youtube

Is Nh2 Polar Or Non Polar Amide Ion In 2021 Nh 2 Molecules Electrons

Why Is Water A Polar Molecule Polarity Of Water Water Molecule Molecules
Molecular Geometry Ap Chemistry

Is Co Polar Or Nonpolar Carbon Monoxide In 2021 Carbon Carbon Monoxide Polar

Is Sf4 Polar Or Non Polar Sulfur Tetrafluoride In 2021 Math Equations Chemical Formula Molecules
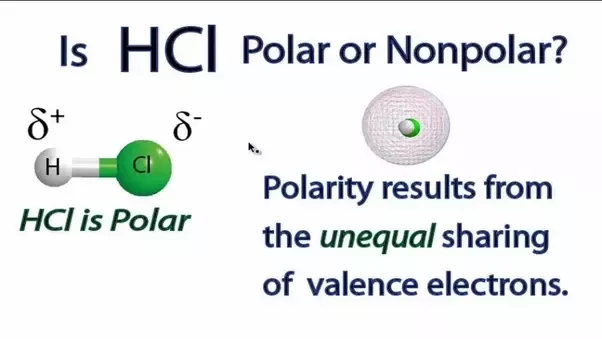
Is Hcl Considered Polar Or Nonpolar Quora
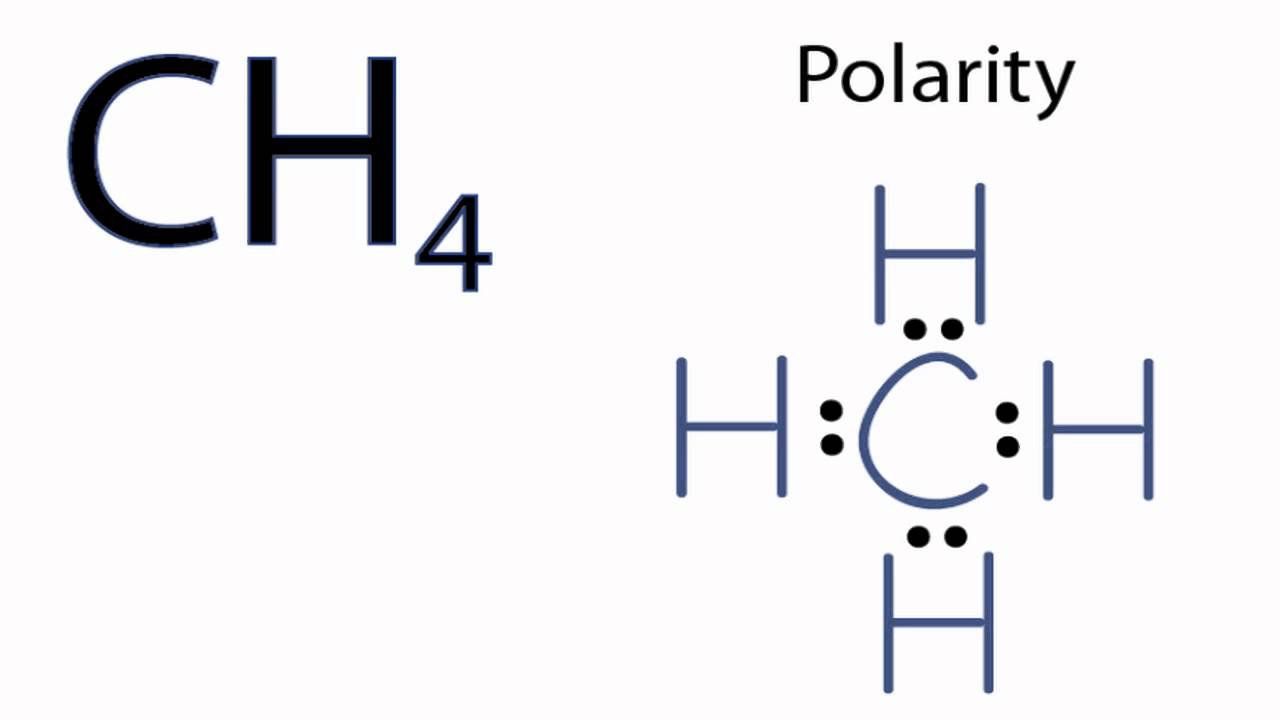
Is Ch4 Methane Polar Or Nonpolar Youtube



