Lewis Dot Structure For N2
The number written at the start refers to the number of molecules and the number written in the subscript refers to the numbers. Lewis dot structure for n2.

The Science Of Studying Lewis Dot Structure Of N2
We have two Nitrogens.
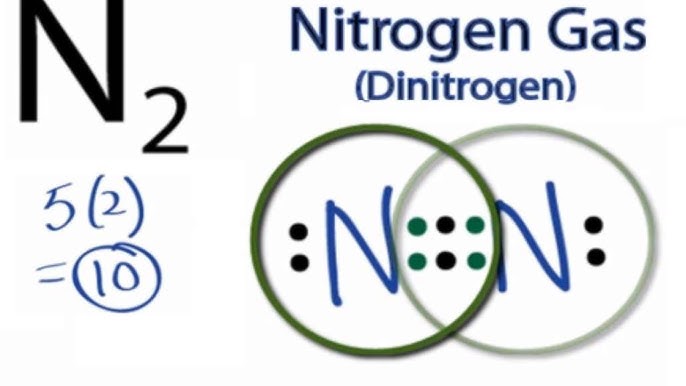
Lewis dot structure for n2. It also is a good example of a molecule with a triple bond. Since N is a member of the Group 5A based on the periodic table the number of electrons in its outermost shell must be 5. Well put the two Nitrogens next to each other and then well put two valence electrons between them to form a chemical bond.
All you want is to offer lewis dot structure of n2 the guidance. In N2 Lewis structuretwo nitrogen atoms has shared six valence electrons and every nitrogen atom has one lone pairs In N2 Lewis structurethere are ten valence electrons. N2 lewis structure bond dot molecular nitrogen molecule does triple valence diatomic electrons atoms lone pair nonmetals occurs orbital bonding.
No lewis dot structure of n2 wonder the workload at schools is extremely high so most of them have no lewis dot structure of n2 other option but to delegate their duties to professional writers. We have a total of 10 valence electrons. Nitrogen N 2 is a commonly tested Lewis structure due to its importance on Earth about 78 of the Earths atomsphere is N 2It also is a good example of a molecule with a triple bond.
The total number of valence electrons between the two N atoms is 10 e-. Utilizing an essay writing system you can hire a matter professional to finish your paper. In the Lewis structure of the N2 molecule there is a formation of a triple covalent bond represented by three lines between two atoms of Nitrogen.
Lewis dot structures reflect the electronic structures of the elements including how the electrons are paired. The N 2 Lewis structure shows two nitrogen atoms bonded in the same way to each other. If the bond energy for the NN bond is 946 kJmol how much energy is needed to break all the bonds in 30 mol of nitrogen molecules.
What is the lewis dot structure for n2. Of system as you could realize these. N2 Lewis Structure Answer.
N2 lewis structure nitrogen dot shape draw electrons rule polar nonpolar valence complete molecules slidesharefile hydrogen octet. Structure lewis n2 diatomic dot nitrogen element diagram ch2o elements electron gas hard. Generally small symmetric molecules are nonpolar.
How many total valence electrons are in N2. Also for the structure to be correct it must follow the octet rule eight electrons per atom. How many total valence electrons are in N2.
The following is the Lewis Dot Structure for N2 2N and N2 are basically two forms of the same element. What is the Lewis dot structure for N2 - 14185321 mircat12 mircat12 12132019 Chemistry Middle School answered What is the Lewis dot structure for N2 2 See answers. In N2 Lewis structuretwo nitrogen atoms has shared six valence electrons and every nitrogen atom has one lone pairs In N2 Lewis structurethere are ten valence electrons.
Multiply those together we have a total of 10 valence electrons for the N2 Lewis structure. Here is the electron dot structure for a single N atom. 2N refers to two molecules of Nitrogen atom and N2 states that two atoms of Nitrogen are present in a single molecule.
Chemical Principles for Organic Chemistry-Robert Boikess 2014-01-01 Covering all the concepts that carry. Draw the Lewis structures of all the molecules involved in the reaction. Therefore N 2 is a nonpolar substance.
This Update includes CNN Videos free with every new copy of the text and is now paperbound at the same low price. N2 Lewis structurenitrogen electron dot structure is that type of structure where we show the total ten valence electrons of N2 as dots or dots and dashes-In Lewis structureit is common that a bonding pair of two electrons can be shown by dash- or dots but a lone pair of two electrons is shown by dots. How_to_do_lewis_dot_structure_for_n2 413 How To Do Lewis Dot Structure For N2 the book is always contemporary always fascinating.
The leftover two 2p orbitals become two π bonds and electrons making a pair between the nitrogen atoms will make a sigma bond. There is a little difference between the two. N2 lewis structure dot following assignment.
The N 2 Lewis structure indicates that the N 2 molecule is perfectly symmetric. For the N2 Lewis structure we have five valence electrons for Nitrogen--its in group 5 or 15 on the periodic table. A step-by-step explanation of how to draw the N2 Lewis Dot Structure Nitrogen Gas Diatomic NitrogenFor the N2 structure use the.

How To Draw The Lewis Dot Structure For N2 Nitrogen Gas Diatomic Nitrogen Youtube

N2 Lewis Structure Molecular Geometry And Hybridization Techiescientist

Lewis Dot Structure For Nitrogen Atom N Youtube

Which Of The Following Is The Correct Lewis Dot Structure For N2 Photo Above Please Help I Got It Brainly Com
Nitrogen N2 Lewis Dot Structure Youtube

N2 Lewis Structure Easy Hard Science

What Is The Lewis Structure Of N2 Socratic

What Is The Lewis Structure For N2 Nitrogen Gas Quora

How To Draw The Lewis Dot Structure For N2 Nitrogen Gas Diatomic Nitrogen Youtube

N2 Lewis Structure Lewis Dot Structure For Nitrogen N2 Gas Of N2 Gas Youtube

Lewis Electron Dot Diagram Of N2 Slide Share

Lewis Structure Of N2 Nitrogen Gas Youtube

N2 Lewis Structure Easy Hard Science

How To Draw The Lewis Dot Structure For N2 Nitrogen Gas Diatomic Nitrogen Youtube

Lewis Structure Of N2 Nitrogen Gas Youtube

What Is The Lewis Structure Of N2 Socratic

N2 Lewis Structure Lewis Structure N2 Hnd Assignment
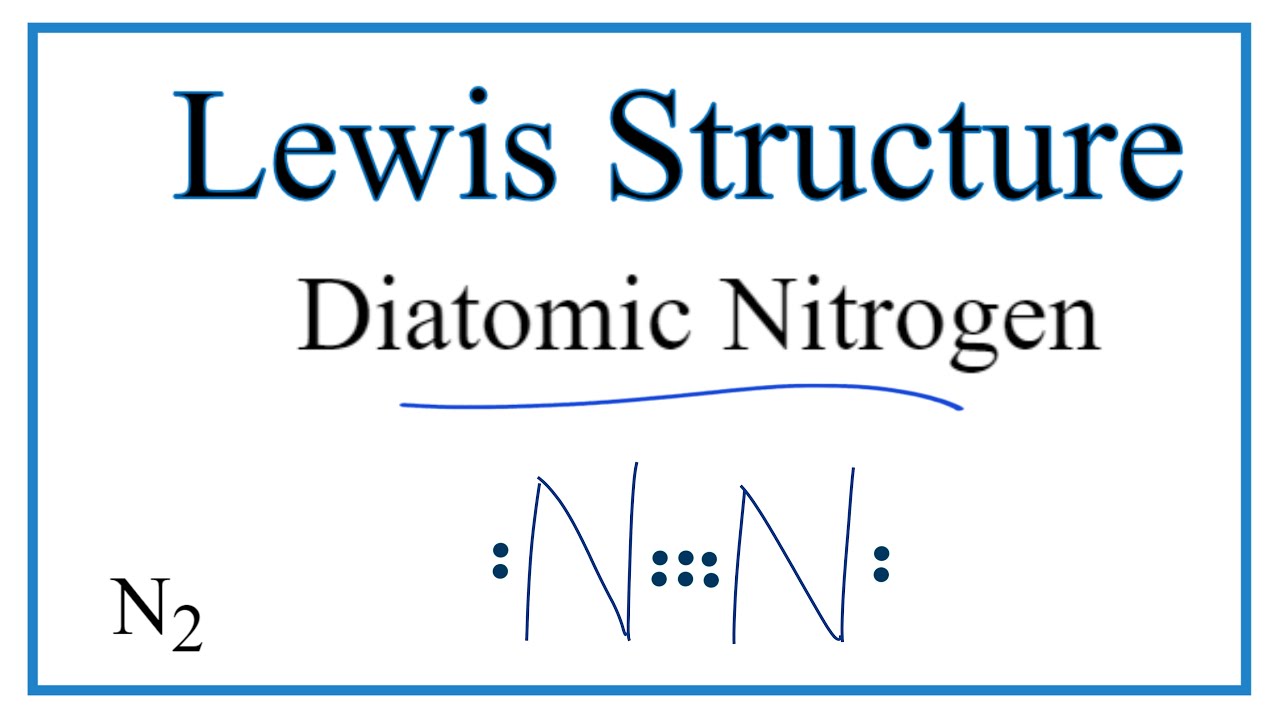
How To Draw The Lewis Dot Structure For Diatomic Nitrogen N2 Youtube
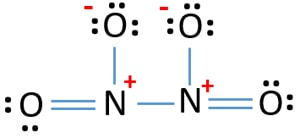
Lewis Structure Of N2o4 Dinitrogen Tetroxide Drawing Steps