Lewis Structure For C2h2br2
A step-by-step explanation of how to draw the C2H2Br2 Lewis Dot Structure 12-DibromoethyleneFor the C2H2Br2 structure use the periodic table to find the. Draw a structure.

Top 12 Mistakes To Avoid When Building Your Barndominium Barndominium Barn House Plans Metal Building Homes
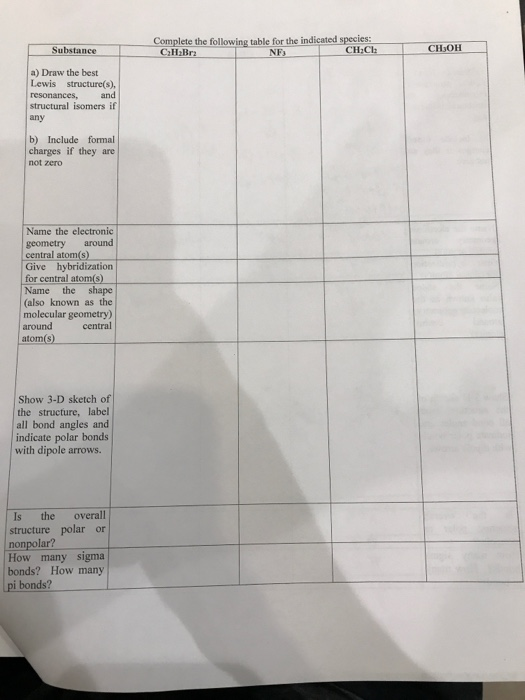
In each case use your Lewis structure to determine the geometry and match this geometry with the correct model.

Lewis structure for c2h2br2. SeO2 is angular and has an AB2 geometry. The -ene means we have the double bond here between the Carbons and the Bromines are on the first and second Carbon in this structure. N2O BI3 H3O NOF IF2 PO43 HCO3 HClO3 CBr2F2 CH2O2 C2F2 C2H3Cl3 C2H2Br2 For All Of These Moleculesions Listed Above.
By signing up youll get thousands of. A Lewis Structure is a simplified representation of the valence shell electrons in a molecule. For C 2 H 2 you have a total of 10 valence electrons to work with.
Experts are waiting 247 to provide step-by-step solutions in as fast as 30 minutes. For c 2 h 2 you have a total of 10 valence electrons to work with. C 2 H 2.
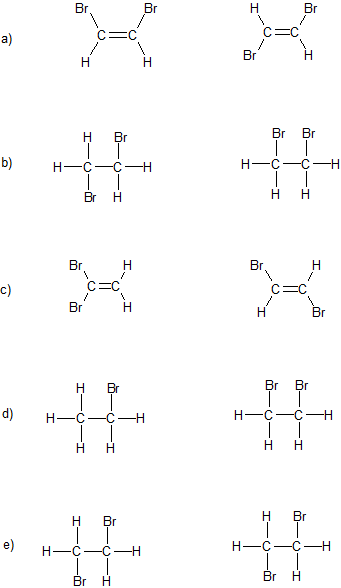
Draw The Lewis Structures For The Species Including All Isomers Andor Resonance Forms. Since the steric number is 4 and there are two lone pairs water has bent geometry. Include H atoms Nonpolar C2H2Br2 one isomer Polar C2H2Br2 two isomers B isomer of the trans isomer The cis isomer is a stereo The isomer that is neither cis nor trans is a structural isomer of.
6 rows 2-Bromoethenyl C2HBr CID 136381 - structure chemical names physical and chemical. If both Bromines were on the first Carbon here we would call it 11-Dibromoethene. Draw the Lewis structure for the molecule.
So thats the Lewis structure for C2H2Br2. The half-life for the reaction in air with hydroxyl radicals is estimated to be to be 28 days for the cis-isomer and 25 days for the trans-isomer of 12-dibromoethyleneSRC calculated from their rate constants of 38X10-12 and 43X10-12 cu cmmolecule-sec at 25 CSRC respectively determined using a structure estimation method3. One of them has no net dipole moment but the other two do.
For this C2H2Br2 Lewis structure we really should call it 12-Dibromoethene. Want to see this answer and more. Draw the Lewis structure for C2H2Br2 and state its molecular geometry.
Cis isomer is a isomer of the trans isomer. This problem has been solved. There are 3 different possible isomers of a dibromoethene molecule C2H2Br2.
For Nonpolar C2H2Br2 one isomer. Median response time is 34 minutes and may be longer for new subjects. Is it polar or nonpolar.
So thats the lewis structure for c2h2br2. Determine the central atom in this molecule. For this C2H2Br2 Lewis structure we really should call it 12-Dibromoethene.
Response times vary by subject and question complexity. One of them has no net dipole moment but the other two do. Draw Lewis structures for each of these isomers.
The total number of bonds formed by sulfur with two oxygen atoms is four. Carbon EN 25 is less electronegative than Bromine EN 28 and hydrogen can only make 1 bond so carbon is the central atom. Draw Lewis structures for each of these isomers.
Calculate the total number of valence electrons present. In order to solve this problem draw the Lewis structure for each of the listed molecules. Get an answer for There are 3 different possible structures known as isomers for a dibromoethene molecule C2H2Br2.
There are 3 different possible isomers of a dibromoethene molecule C2H2Br2. One of them has no net dipole moment but the other two do. Electrons are shown as dots or for bonding electrons as a line between the two atoms double line for a double bond.
Structure properties spectra suppliers and links for. It shows arrangement of the electrons around individual atoms in a molecule. The solution is to share three pairs of valence electrons and form a triple bond between the Carbon atoms in C 2 H 2.
The molecular geometry of C2H2Br2 is trigonal planarThe molecular geometry of C2H2Br2 is trigonal planar. In drawing the Lewis structure for C 2 H 2 also called ethyne youll find that you dont have enough valence electrons available to satisfy the octet for each element if you use only single bonds.

7 Toxic Organic Chemicals Ppt Download

Chemistry Notes Chemical Bonding Lewis Structures Vsepr Theory Vsepr Theory Chemistry Notes Covalent Bonding

Help Students Understand How To Draw Lewis Structures Understand Molecular Geometry And The Polar Nature Of Ionic And In 2021 Molecular Shapes Vsepr Theory Molecular
Chemical Bonding Pdf Chemical Polarity Ion
Substance Complete The Following Table For The Chegg Com

Bethany Vineyard Winery On Instagram Natural Views Outdoor Indoor Ceremony
2 7 Isomerism Introduction Chemistry Libretexts

2 7 Isomerism Introduction Chemistry Libretexts

Rank From Largest Dipole To Smallest Dipole Youtube

H2o2 Lewis Structure How To Draw The Dot Structure For H2o2 Molecular Geometry Molecular Shapes Intermolecular Force
Chemical Bonding Pdf Chemical Polarity Ion

Rank From Largest Dipole To Smallest Dipole Youtube

A Bond Will Form If Valence Bond Theory Ppt Download

The Molecule Xef4o Has A Central Xenon Atom With Four Fluorines And An Oxygen Course Hero

2 7 Isomerism Introduction Chemistry Libretexts

A Bond Will Form If Valence Bond Theory Ppt Download

Eeoc Versus Global Horizons Et Al



