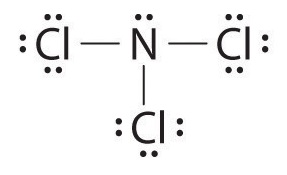
Lewis Structure For Ncl3
Draw the Lewis structure for the molecule. The total number of lone pairs in NCl3 is.
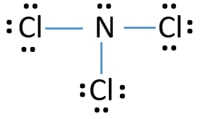
Ncl3 Nitrogen Trichloride Lewis Structure
Then learn how to predict the shape of a molecule by applying the VSEPR theory to the Lewis dot structure.
Lewis structure for ncl3. Learn how to draw a Lewis Structure and how to use it to assign a molecules shape geometryAre you taking a class with me. NCl3 lewis structure is the same as the NF3 structure. Predict giving a reason the Cl - N - Cl bond angle in NCl3.
Nitrogen trichloride or NCl3 is arranged in a tetrahedral structure with one single lone pair located on the Nitrogen atom. NCl3 is an AX3E system and like NH3 trigonal pyramidal So Ill go ahead and draw in the lone pair of electrons on the nitrogen. The Lewis structure for NCl3 is.
An electron from the 22 orbital and three other electrons from 2p orbitals participate in forming bonds. It contains one nitrogen atom at the center and three chlorine atoms spaced evenly around it. What Is The ARRANGEMENT For This Structure.
Now lets see the proper steps to draw a lewis structure-1. Draw the Lewis structure of NCl3 HCN OF2 and CO2. In the NH 3 Lewis structure Nitrogen N is the least electronegative so it goes in the center.
Hence the Geometry of the molecule of NCl3 is Trigonal pyramidal. Three pairs will be used in the chemical bonds between the N and Cl and one pair of. Unlike protons and neutrons valence electrons take part in the excitement of a chemical reaction.
Lewis structure of NCl3 can be drawn by using valence electrons of nitrogen and chlorine atoms. Chemistry QA Library Draw the Lewis structure of NCl3 HCN OF2 and CO2. Determine the central atom in the molecule.
It most certainly is it has a similar structure to NI3 MOTM Dec 2001 and is even more unpredictably explosive. There is one lone pair on nitrogen atom and three lone pairs on each chlorine atom. In the Lewis structure for NCl 3 there are a total of 26 valence electrons.
What is the hybridization of the nitrogen atom. Draw the Lewis structure of NCl3 HCN OF2 and CO2. The N-Cl bonds are formed by the overlap of the ____ hybrid orbitals on nitrogen with the _____ orbitals on Cl.
NCl3 from the air environment reacts with DPD 3 releasing iodine which reacts with DPD 1 and produces a coloration proportional to the amount of NCl3 from the sampled indoor swimming pool air. One lone pair is present on the central atom of the NCl3 lewis structure and three lone pairs on each chlorine atom. Find the total number of valence electrons in a molecule- Adding up the valence electrons of all the atoms in a molecule is the first step.
First week only 499. Related to this Question. When we consider both lone pairs and bond pairs we are referring to the Structure of the molecule.
Now the central atom is generally the least electronegative atom or atom with the. Nitrogen trichloride NCl3 lewis structure contains three N-Cl bonds. What are its electron-pair and molecular geometries.
Draw The Lewis Structure. Draw The Lewis Structure For NCl3. P STEM Educatormr_piasecki Moramoratheexplora22 sarah coffeyscoffeyx Micarimissmicari GYPSEAgypseasoul.
Hence the Structure of the molecule of NCl3 is Tetrahedral since a lone pair of electron can be considered to be a bond pair. Lewis dot structures are useful to predict the geometry of a molecule. NCl 3 is similar to NH 3 and NF 3.
Watch popular content from the following creators. Also there are no charges on atoms in NCl3. Include lone pairs NCl3.
Our sampling of the monitored swimming pool environments evidenced a mean NCl3 level 637-220 ugcu m higher than the recommended WHO value 500 ug. Ncl3 lewis structure 1624M views Discover short videos related to ncl3 lewis structure on TikTok. What orbitals on N and Cl overlap to form bonds between these elements.
40-10 30e-15 lone pairs. To do that we need to do the following steps. Lewis Dot Structure For The Covalent Compound Ncl3 electron geometry pf3 ncl3 lewis structure dot draw structural lewis structures nitrogen electrons structure chlorine oxygen bonding chemical pairs lone pair chemistry drawing al libretexts three each around place lewis dot structure ncl3 nitrogen trichloride draw write chemistry lewis compound ionic structures unit formula chapter.
Calculate the total number of valence electrons present. Drawing the Lewis Structure for NCl 3. Choose a central atom and draw a skeletal structure- Sketch a skeletal of the molecule with only single bonds.
Our mission is to help you. If you can do those Lewis structures NCl 3 will be easy. Start your trial now.
Draw the Lewis structure of NCl3. If so feel free to rate me ath.

Draw The Lewis Structure Of The Following Molecule Include Lone Pairs Ncl3 Brainly Com
Ncl3 Lewis Structure Studyrankersonline

How To Draw Lewis Structure For Ncl3 Drawing Easy

Determine The Electron Geometry Eg And M Clutch Prep

Ncl3 Lewis Structure How To Draw The Dot Structure For Ncl3 Youtube
Answer In General Chemistry For Brittany Wallace 98925

Choose A Lewis Structure For Ncl3 Clutch Prep

Draw The Lewis Dot Structure For Ncl3 Clutch Prep

Choose A Lewis Structure For Ncl3 Clutch Prep

Ncl3 Lewis Structure And Molecular Geometry Geometry Youtube
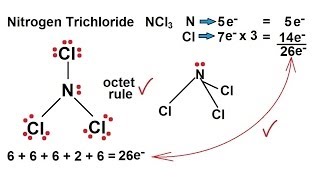
Chemistry Chemical Bonding 14 Of 35 Lewis Structures Nitrogen Trichloride Ncl3 Youtube
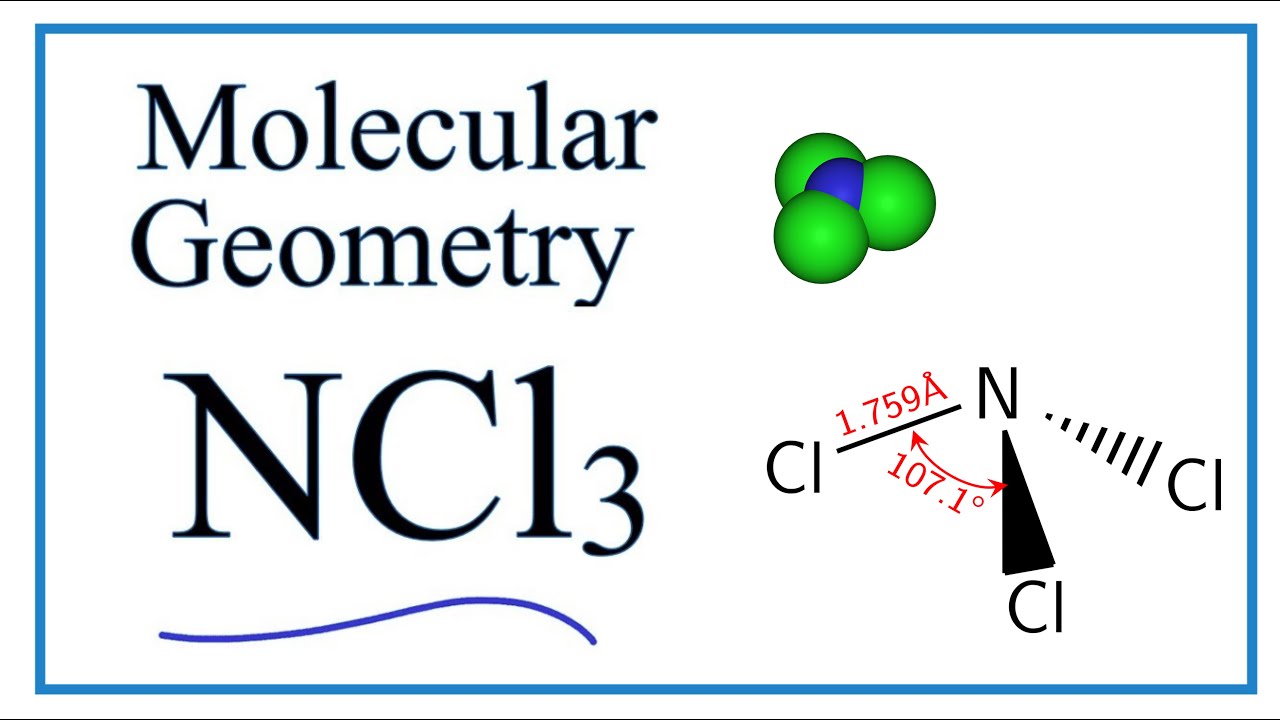
Ncl3 Molecular Geometry Shape And Bond Angles Youtube

Lewis Structure Of Ncl3 Nitrogen Trichlorode Youtube
Is Ncl3 Polar Or Non Polar Quora

Ncl3 Lewis Structure Molecular Geometry Polar Or Nonpolar Bond Angle

The Total Number Of Lone Pairs In Ncl3 Is Study Com
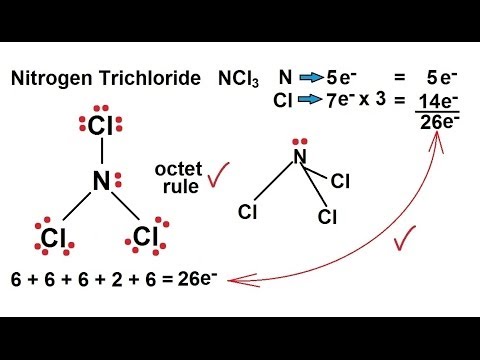
Chemistry Chemical Bonding 14 Of 35 Lewis Structures Nitrogen Trichloride Ncl3 Youtube

Ncl3 Lewis Structure How To Draw The Dot Structure For Ncl3 Youtube