Lewis Structure Of Sif6 2-
However when you remove an electron from it making it NO2 the molecule becomes linear due to the loss of a lone electron. Hexafluorosilicic acid has a distinctive sour taste and pungent smell.
Question 5 2 F F Si F F What Is The Chegg Com
Why Can This Compound Be Formed Using S But Not O As The Central Atom.

Lewis structure of sif6 2-. Hence this is the right Lewis structure of SF6. Hexafluorosilicic acid is an inorganic compound with the chemical formula H 2SiF 6 also written as 2SiF 6. O 2-7B M a The most likely Lewis structure for BF4-is drawn below.
List molecules polar and non polar. I quickly take you through how to draw the Lewis Structure of SF6 2- Silicon HexaFluoriode Ion. BF F F F Four bonding pairs of electrons surround the central boron atom in this structure.
Ill tell you the polar or nonpolar list below. A single chemical bond. A step-by-step explanation of how to draw the SiF62- Lewis Dot StructureFor the SiF6 2- Lewis structure calculate the total number of valence electrons for.
Be sure to put brackets and a 2- around the SiF 6 2-Lewis structure to show that it is an ion. On the other hand nitrogen dioxide NO2 is an AX2E species and it has an angle of. For the SF6 Lewis structure there are a total of 12 valence electrons on the Sulfur S atom.
Write the skeletal structure of so that least electronegative atom as central atom. This problem has been solved. It is a colorless liquid mostly encountered as diluted aqueous solution from there the second chemical notation also proposed.
There are a total of 48 valence electrons in the Lewis structure for SF6. Here as Sulphur is sharing its electrons with the Fluorine atoms we will look at its hybridization. The VSEPR predicts the Octahedral shape.
While the Lewis Structure is a 2-dimensional depiction of an atom of a molecule molecular geometry is the visualization and designing of the atoms in a 3-dimensional space. The Sulphur atom has sp3 Hybridization and the bond angle of F-S-F is 98 degrees. There are a total of 48 valence electrons in SiF 6 2-.
Silicon Si is the least electronegative and goes at the center of the Lewis structure. Silicon Si is below Period Two on the periodic table and can hold more than 8 valence electrons. Out of 48 electrons 12 electrons are used in skeletal structure and remaining electrons are distributed as lone pairs on terminal atoms so that all atoms satisfied its octet.
SF2 has a simple Lewis structure in which the Sulphur atom is in the centre forming single bonds with both the Fluorine atoms. SiF 6 2-is d 2 sp 3 hybridized and contains no lone pair and 6 bonding pairs of valence electrons around the Silicon. Hexafluorosilicate 2- is a silicon coordination entity.
SiF6 2- is Polar. SF5 arranges 5 pairs of electrons in a trigonal bipyramidal structure. This arrangement gives the boron atom a complete octet and a formal charge of -1.
Contact may irritate skin eyes and mucous membranes. Note that Sulfur S is in Period 3 on the periodic table and can have an expanded octet and is able to have more than 8 valence electrons. The concept of molecular geometry aims to depict the generic shape and structure of a molecule accurate to the length between different bonds the bond and torsional.
Used to make other chemicals. The VSEPR predicts the Octahedral shape. Orthosilicate O4Si-4 CID 104812 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more.
NO2 is a bent molecule. Thus F has 7 valwence electrons and Si has 4. If you want to quickly find the word you want to search use Ctrl F then type the word you want to search.
Why can this compound be formed using S. SF6 is an octahedral shape which makes perfect sense. Construct The Lewis Structure Model For The Covalent Compound Sulfur Hexafluoride SF6.
Elements in the first 2 periods of the Periodic Table do not have access to the d sublevel and must adhere to the octet or duet H and He rule. The electronic configuration of SF6 in its ground state is 3s23p4. There are two lone pairs of electrons on the Sulphur atom which makes the geometry of the molecule bent.
Public health information CDC Research information NIH SARS-CoV-2 data NCBI Prevention and. Appears as a crystalline solid or the solid dissolved in a liquid. For the SF6 Lewis structure you should take formal charges into account to find the best Lewis structure.
Quiz your students on SiF6 2- Dot Lewis Structure Molecular Geometry Bond Angle Polar or Nonpolar using our fun classroom quiz game Quizalize and personalize your teaching. It is manufactured as a coproduct in the. Flourine is in 7A in the Periodic Table and Silicon in 4A.
Is no2 linear or bent. Construct the lewis structure model for the covalent compound sulfur hexafluoride SF6. The Lewis structure for O2-is shown below.
Now that we know the Lewis Structure of SF6 we can now determine the atoms hybridization in the molecule. May be toxic by ingestion. The Lewis structure for anion can be depicted as follows.
It is produced naturally on a large scale in volcanoes. I also go over hybridization shape and bond angle. The purpose of a Lewis Structure is to show by means of dots the un-bonded valence electrons on an atom or molecule.
Count the total number of valence electrons.

How Many Electrons Surround The Central Atom On Sif62 Study Com
Solved Draw A Lewis Structure For Each Of The Following Molecules Or Ions Describe The Electron Pair

What Is The Molecular Shape Of Sif62 As Predicted By The Vsepr Theory A Trigonal Bipyramidal B Seesaw C Hexagonal D Octahedral E Tetrahedral Study Com
Hexafluorosilicate F6si Chemspider
Solved Draw A Lewis Structure For Each Of The Following Molecules Or Ions Describe The Electron Pair

Sif62 Lewis Structure How To Draw The Lewis Structure For Sif6 2 Youtube
How To Draw Lewis Structure For Sf6 Drawing Easy

Wn Lewis Structure For Si2 Molecular Geometry And Hybridization

Sif62 Lewis Structure How To Draw The Lewis Structure For Sif6 2 Youtube
Chemical Education Journal Cej Vol 13 No 2 Registration No 13 19

What Is The Molecular Geometry Of Sif62 As Predicted Chegg Com
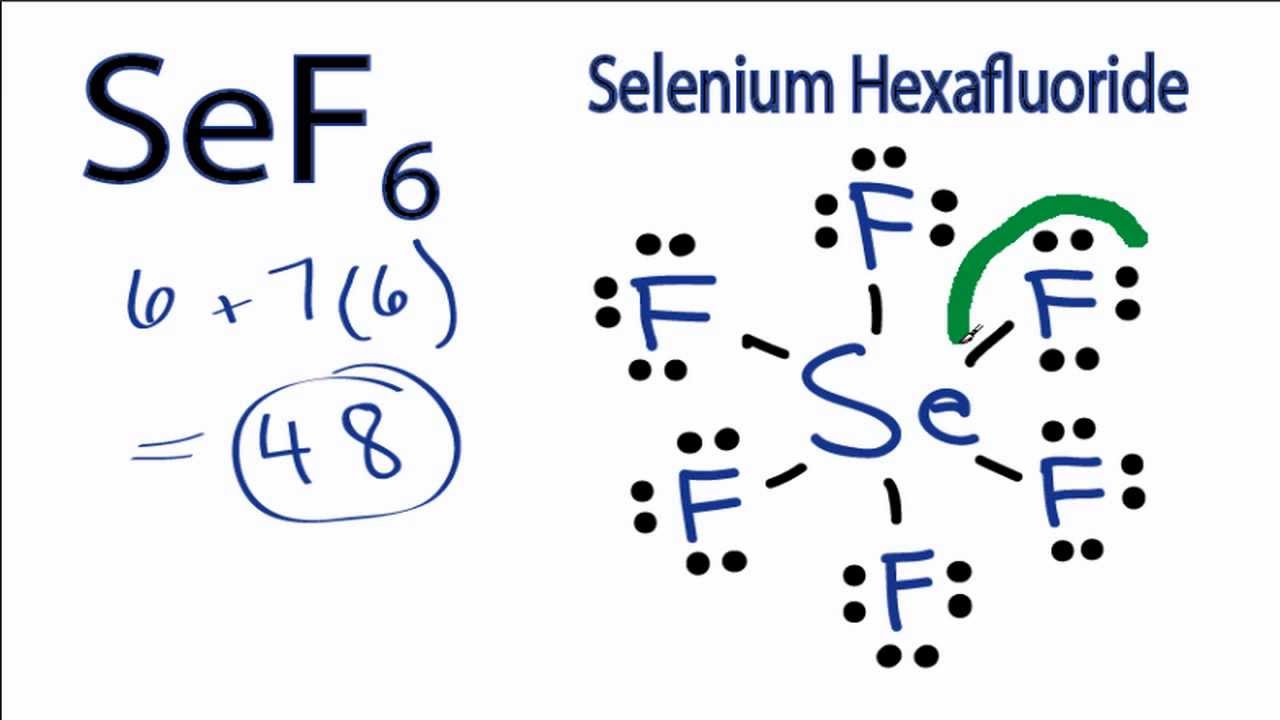
Sef6 Lewis Structure How To Draw The Lewis Structure For Selenium Hexafluoride Youtube
Kawbikfdwq Sif6 2 Lewis Structure Example
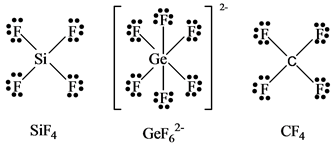
Solved Silicon Tetrafluoride Reacts With F To Produce The Hexafl Chegg Com

Sif62 Lewis Structure How To Draw The Lewis Structure For Sif6 2 Youtube

Sf6 Molecular Geometry Lewis Structure Shape And Polarity
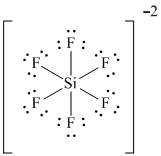
Solved Write A Lewis Formula For The Anion Sif6 2 That Would Be Chegg Com

Lewis Dot Of The Silicon Hexafluoride Ion Sif6 2
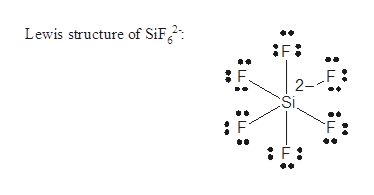
Answered Sif62 Nsf F2co Fno2 Deduce The Bartleby
