N2 Lewis Structure Triple Bond
In N2 Lewis structureboth nitrogen follow the octet rule. Each N is surrounded by two dots which are called lone pair of electrons.

N2 Lewis Structure Easy Hard Science
The N2 molecule is diatomic meaning that two atoms of the same element are connected in a pair.

N2 lewis structure triple bond. There is a triple bond between both nitrogen atoms. Each nitrogen atom is surrounded by a lone pair of electrons. In the Lewis structure of the N2 molecule there is a formation of a triple covalent bond represented by three lines between two atoms of Nitrogen.
According to the octet rule nitrogen atoms need to bond three times. The N2 Lewis structure has a triple bond between two nitrogen atoms. The N2 molecule is diatomic meaning that two atoms of the same element are connected in a pair.
According to the octet rule nitrogen atoms need to bond three times. We used a triple bond to give octets to both of the Nitrogens and still use 10 valence electrons. Each N is surrounded by two dots and three sticks or lines representing another 6 electrons in the N2 triple bond.
With the Lewis structure for. How many total valence electrons are in N2. The Lewis structure for Nitrogen is as follows You can see the triple bond with the lone pair on both of the nitrogen atoms.
Carbon forms one single bond with the Hydrogen atom and forms a triple bond with the Nitrogen atom. N2 Lewis structure would comprise of two atoms of Nitrogen N atoms. A step-by-step explanation of how to draw the N2 Lewis Dot Structure Nitrogen Gas - Diatomic NitrogenFor the N2 structure use the periodic table to find t.
But now the Nitrogen on the left also has a full octet and were only using the 10 valence electrons that we have for the N2 Lewis structure. The N2 molecule is diatomic meaning that two atoms of the same element are connected in a pair. The two letter Ns in the N2 Lewis structure represent the nuclei centers of.
Express your answer as a whole number. Then each N atom has a lone pair placed on it. The molecular orbital diagram for nitrogen is as follows You can see the accounting for each of the valence electrons 5 from each atom place.
Draw the Lewis Dot structure for N2 on paper not on Canvas then answer the questions. How many triple bonds are in. Since there are only two regions of electron density 1 triple bond 1 lone pair the hybridization must be sp.
The N2 Lewis structure has a triple bond between two nitrogen atoms. According to the octet rule nitrogen atoms need to bond three times. The Lewis structure for N2 contains Group of answer choices a double bond.
The molecular geometry of N 2 is linear. So each N is surrounded by 8 total valence electrons giving it an octet and making it stable. The N2 Lewis structure has a triple bond between two nitrogen atoms.
How many double bonds are in N2. The structure of N2 has two N atoms triple bonded to each otherlinearly. Lewis Structure Of N2- Key Points.
Number of electrons in the valence shell of nitrogen atom 5. How to Draw the Lewis Structure of N2 - with explanationCheck me out. These lone pairs unbonded electrons are representing another 6 electrons in the N2 triple bond.
It has a triple bond and one lone pair on each nitrogen atom. HCN has a total of 10 valence electrons. Express your answer as a whole number.
It is covered under AX2 molecular geometry and has a linear shape. N2 is colorless odorless and tasteless gas. The leftover two 2p orbitals become two π bonds and electrons making a pair between the nitrogen atoms will make a sigma bond.
In the lewis structure of N2 there is a triple bond between two nitrogen atoms. In N2 Lewis structurewe get five pairs of electronsOut of five pairs of electrons N2 has three bond pairs and two lone pairs of electronsHereeach nitrogen atom carries one lone pair of electrons. Hybridization of Nitrogen N2.
The bond angles of HCN is 180 degrees. How many single bonds are in N2. You do not have the required permissions to view the files attached to this post.
When determining hybridization you must count the regions of electron density.

How To Draw The Lewis Dot Structure For N2 Nitrogen Gas Diatomic Nitrogen Youtube

Lewis Structure Of N2 Nitrogen Gas Youtube

Lewis Dot Structure For Nitrogen Atom N Youtube
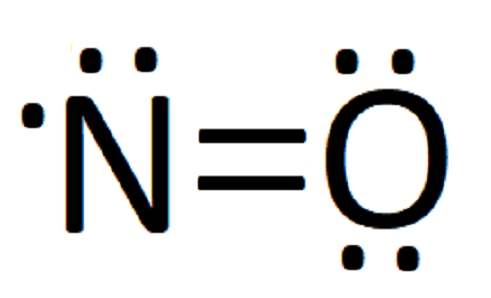
No Lewis Dot Structure Science Trends

Lewis Dot Structure Easy Hard Science

What Is The Lewis Structure Of N2 Socratic

N2 Lewis Structure Easy Hard Science
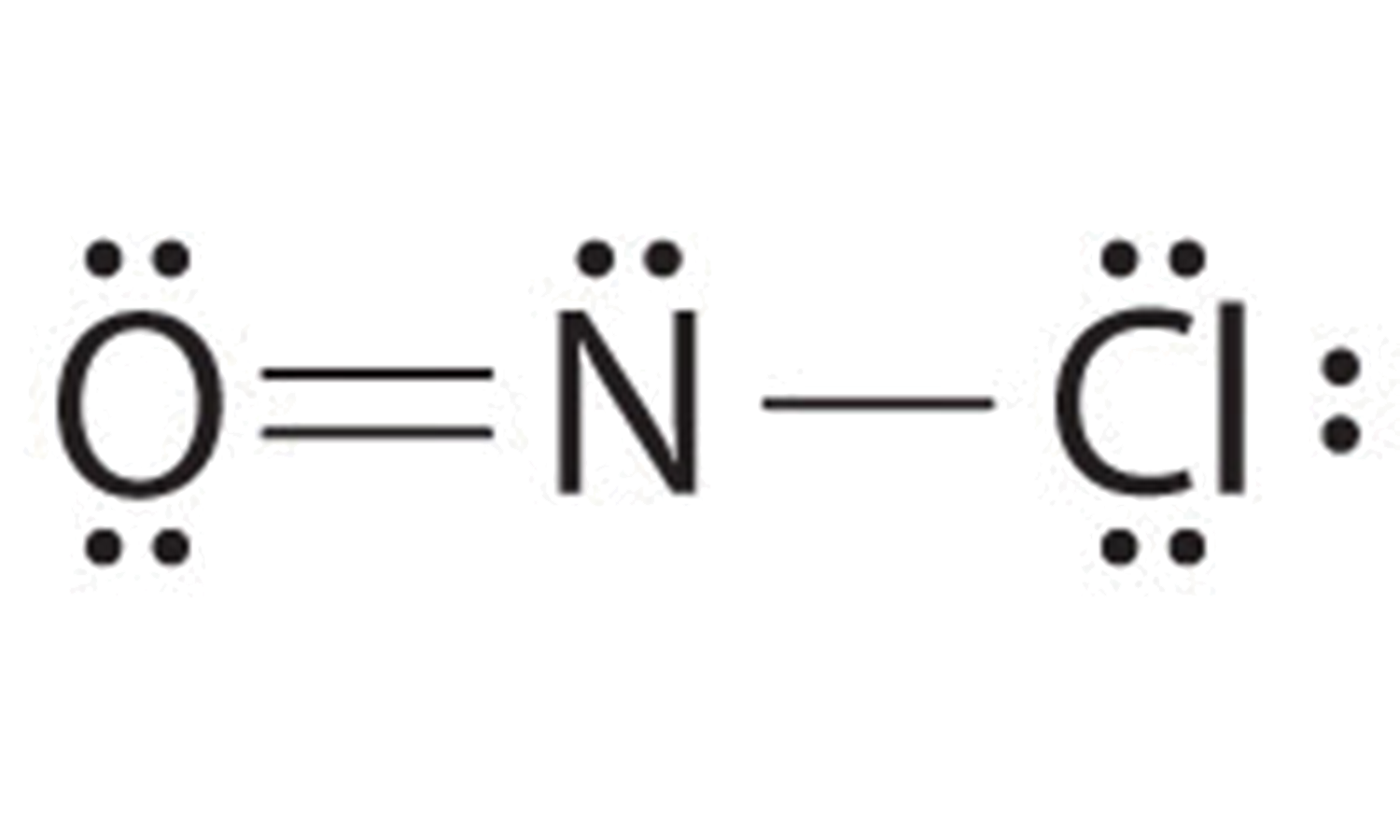
9 5 Covalent Bonding Lewis Structure Chemistry Libretexts

Lewis Dot Structure Easy Hard Science

How To Draw The Lewis Dot Structure For N2 Nitrogen Gas Diatomic Nitrogen Youtube
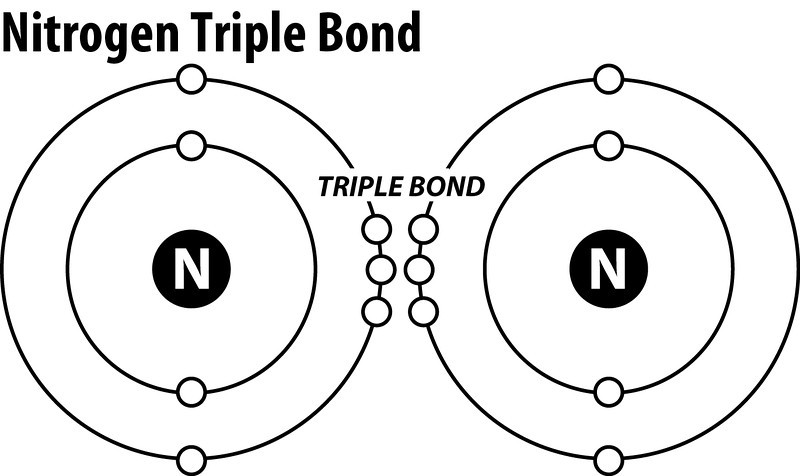
What Is The Lewis Structure Of N2 Socratic

N2 Lewis Structure Lewis Structure N2 Hnd Assignment

Co2 Lewis Structure Carbon Dioxide Youtube

N2 Lewis Structure Molecular Geometry And Hybridization Techiescientist

Lewis Structure Of N2 Nitrogen Gas Youtube

No2 Nitrogen Dioxide Lewis Dot Structure Science Trends

N2 Lewis Structure Molecular Geometry And Hybridization Techiescientist

What Is The Lewis Structure Of N2 Socratic

No Lewis Dot Structure Science Trends