Nho3 Lewis Structure
There are three single bonds and one lone pair of electrons in NH3 molecule. Now lets move forward and realize the electron geometry.

The Lewis Structure Of Hno3 Chemistry Stack Exchange
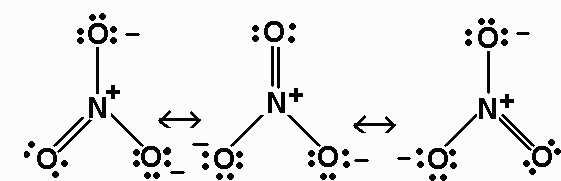
NO3 Lewis Structure Molecular Geometry and Hybridization NO3 is a polyatomic ion with a negative charge.

Nho3 lewis structure. NH3 Lewis structure molecular geometry. Check the formal charges to be sure that each atom has a. In the lewis structure of ammonia NH 3 there are three N-H bonds and one lone pair on nitrogen atomLewis structure of NH 3 can be drawn by starting from valence electrons of nitrogen and hydrogen atoms in several steps.
A step-by-step explanation of how to draw the N2O3 Lewis Dot Structure Dinitrogen trioxideFor the N2O3 structure use the periodic table to find the total. Nitrogen has a steric number of 3 while the oxygen atom in the OH ion has a steric number of 4. Ammonia is lighter than the air colorless and pungent in smell.
The HNO 3 Lewis structure is easier to think of if you consider it NO 3 with an H bonded to one of the oxygen atoms. There are 8 valence electrons available for the Lewis structure for NH 3. It has a role as a protic solvent and a reagent.
Lewis structure of nitric acid There is a NO bond in nitric acid lewis structure. The HNO3 Lewis structure is best thought of as the NO3 with an H attached to one of the oxygen atoms. HNO3 needs a bit more describing its trigonal planar with N as the center NO3 and bent around the O bound to H.
Ammonia is the simplest binary hydride made up of nitrogen and hydrogen denoted by its chemical formulae as NH3. Drawing the Lewis Structure for NH 3 Ammmonia Ammonia NH 3 is a commonly tested Lewis structure due to its widespread use in agriculture as a fertilizer. However the first two resonance structures are significantly more favorable than the third because they have smaller amount of formal charges.
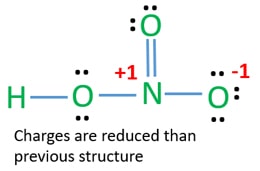
This pair exerts repulsive forces on the bonding pairs of electrons. One group has an unshared pair of electrons. In HNO 3 Lewis structure Nitrogen N is the least electronegative atom and goes in the center of the Lewis structure.
In the Lewis structure of HNO3 the molecule is formed due to the hybridization of two orbitals. Thus Ammonia is an example of the molecule during which the central. The compound has its chemical name as nitrate formed after nitric acid looses a proton from it.
The Lewis dot structure for HNO 3 nitric acid shows how the valence electrons are used in the bonding between atoms. N has tetrahedral electronic geometry. NH3 Lewis Structure Geometry and Hybridization.
ClF5 is square pyramidal. Based on octet rule alone there are 3 possible resonance structures that are favorable. Each step of drawing the lewis structure of NH 3 is explained in detail in this tutorial.
In the lewis structure of nitric acid there is a 1 charge on nitrogen atom and one double bond between nitrogen and one oxygen atom. Nitric acid is a strong oxidizing agent commonly used to add the nitro group. The N atom in HNO3 has SP2 hybridization and the O atom has SP3 hybridization.
How is the Lewis structure for ClF5 and HNO3 written. HNO 3 Nitric acid lewis stricture is drawn step by step by using valence electrons of each element. This is a pattern seen with many acids.
It is a conjugate acid of a nitrate. Nitric acid is a nitrogen oxoacid of formula HNO3 in which the nitrogen atom is bonded to a hydroxy group and by equivalent bonds to the remaining two oxygen atoms. NH3 electron geometry is.
Tetrahedral because its four groups of electrons. For HNO3 in order to satisfy the octet rule the nitrogen atom would form 1 double bond and 2 single bonds. Ammonia NH 3 Lewis Structure Steps of Drawing.
It also is a good example of a molecule with a trigonal prymidal molecular geometry. For the HNO3 Lewis structure calculate the total number of valence electrons for the HNO3 molecule. The shape is distorted because of the lone pairs of electrons.
It has a molecular geometry of trigonal pyramidal which also looks like a distorted tetrahedral structure. So it is also referred to by the name of nitrogen oxoanion. It is a stable pnictogen hydride where all the atoms are covalently bonded to achieve a reactive state.

How To Determine The Molecular Geometry Of Hno3 Quora
Hno3 Nitric Acid Lewis Structure

The Lewis Structure Of Hno3 Chemistry Stack Exchange

The Lewis Structure Of Hno3 Chemistry Stack Exchange
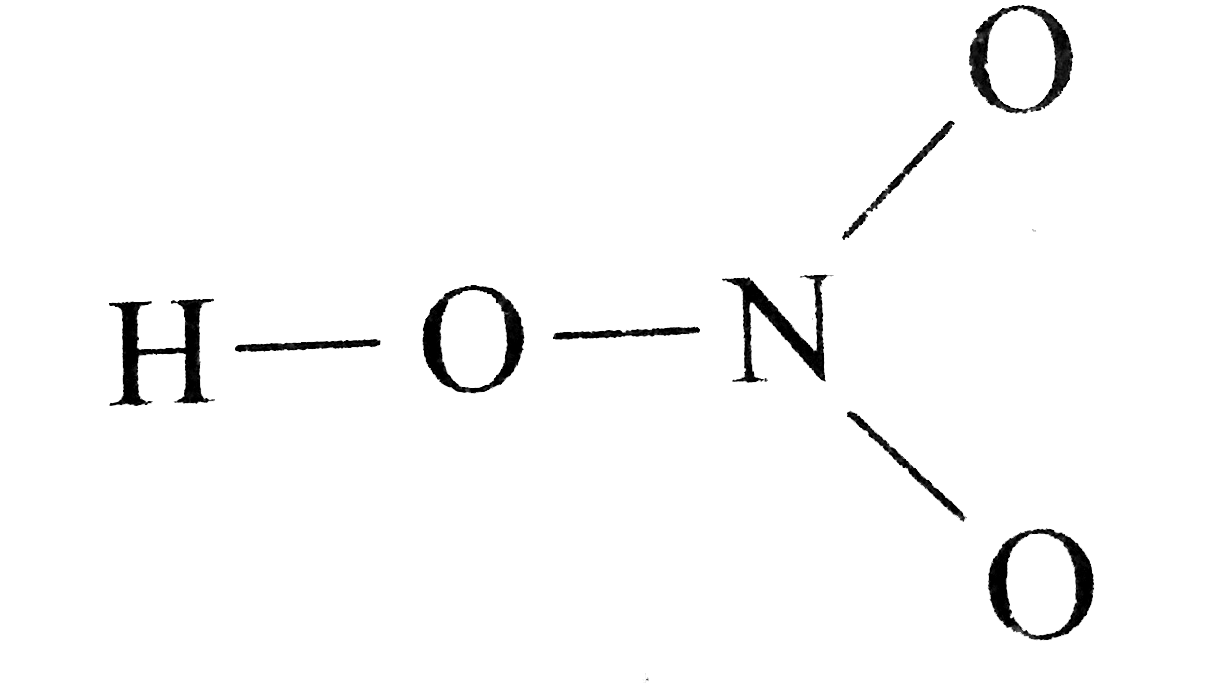
Draw The Lewis Structure Of Nitric Acid Hno 3

What Is Nitric Acid Structure Uses Formula Video Lesson Transcript Study Com
In Lewis Structure Of Hno3 Does Nitrogen Share Two Electrons With Two Of The Oxygens Do Those Oxygens Share Or Not Share Electrons With The Nitrogen Quora
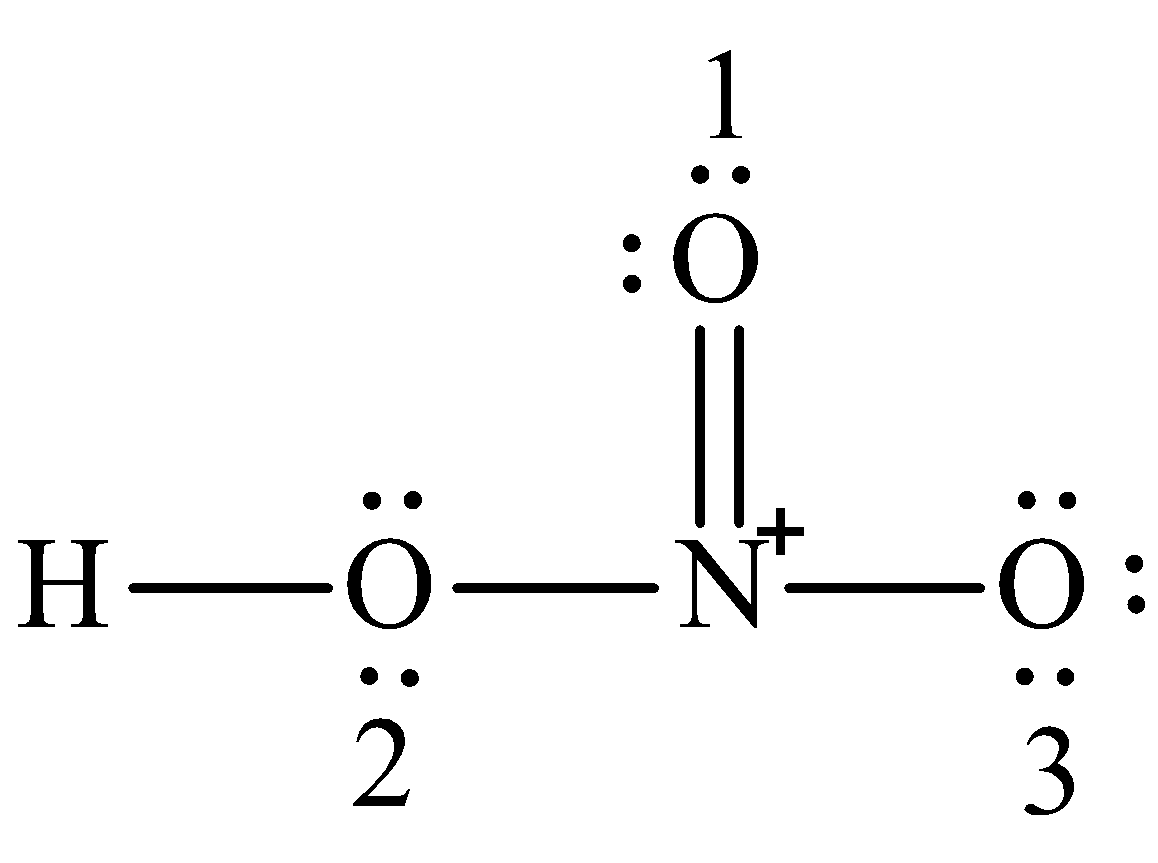
Write Lewis Structure Of The Hno3 And Show Formal Charge Class 12 Chemistry Cbse

Resonance Structures For Hno3 Nitric Acid Youtube

Hno3 Lewis Structure Nitric Acid Youtube

Hno3 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

Write Lewis Structure Of The Hno3 And Show Formal Charge On Each Atom
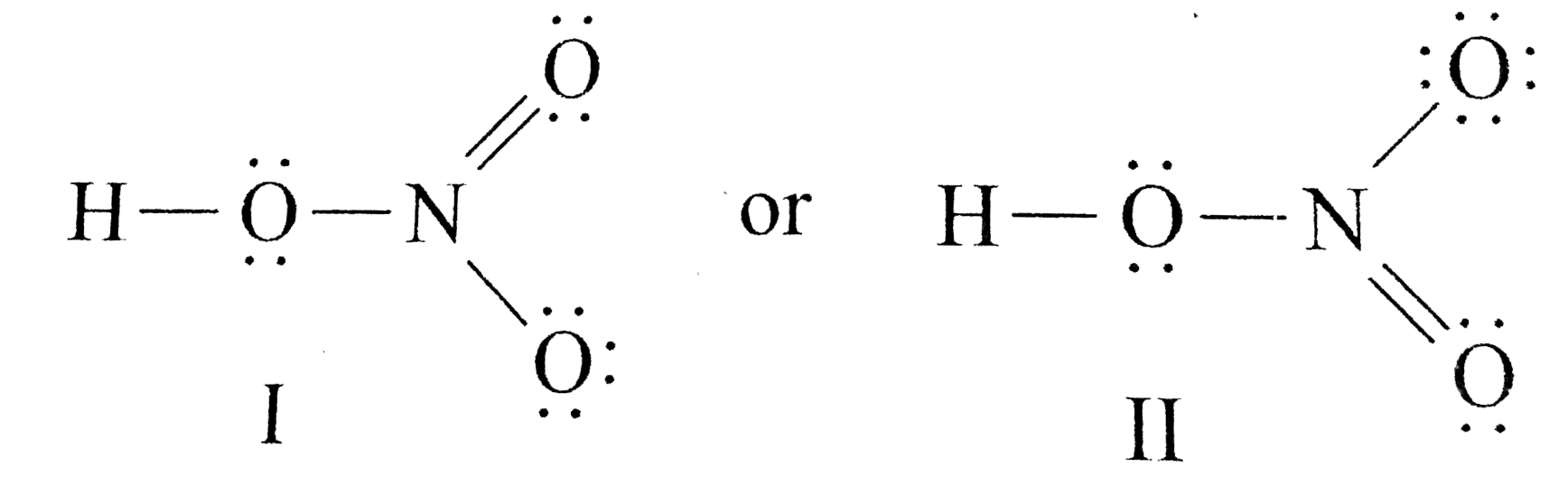
Draw The Lewis Structure Of Nitric Acid Hno 3

How Is The Lewis Dot Structure For Nitric Acid Determined Quora
Hno3 Lewis Structure How To Draw The Lewis Structure For Hno3 دیدئو Dideo

The Lewis Structure Of Hno3 Chemistry Stack Exchange