Pcl3 Electron Geometry
Eg trigonal planar mg tetrahedral polar C. What are PCl3 electron and molecular geometry.

Dublin Schools Lesson Molecular Geometry What Shapes Do Molecules Have
An explanation of the molecular geometry for the AlCl3 ion Aluminum chloride including a description of the AlCl3 bond angles.

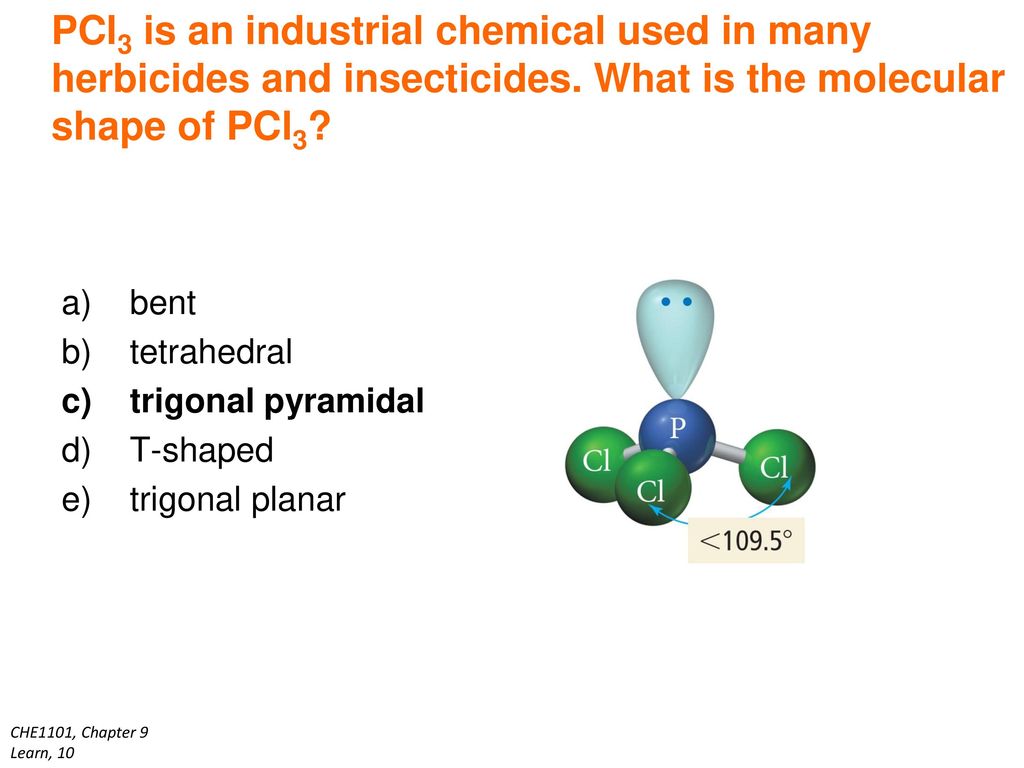
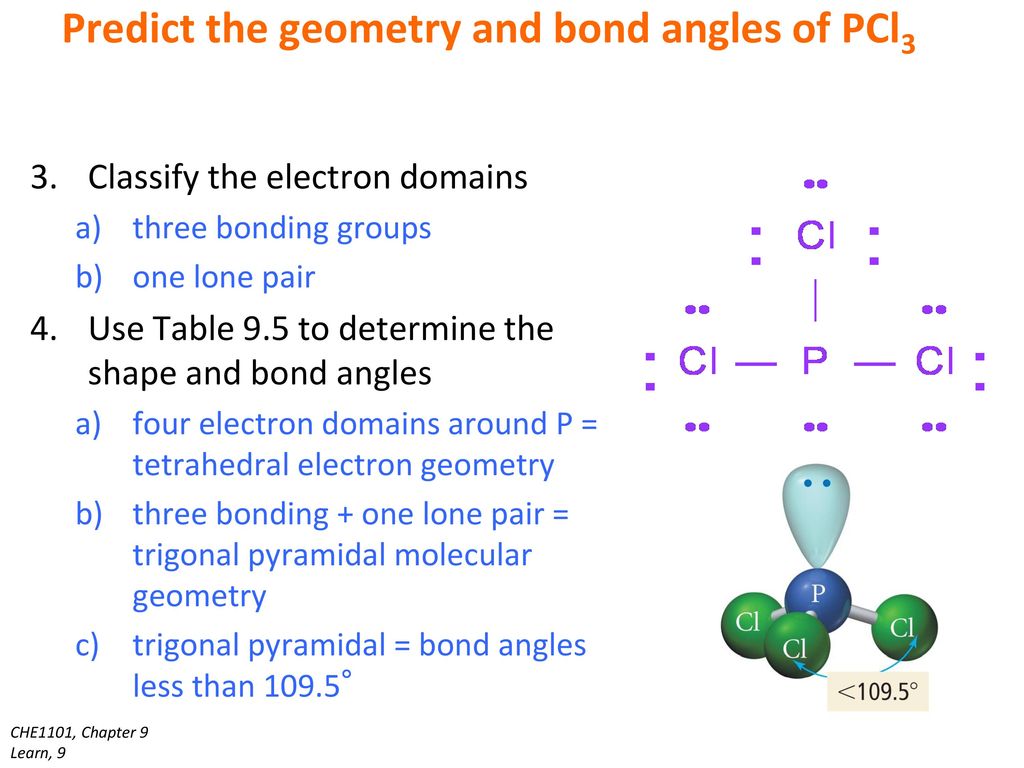
Pcl3 electron geometry. PCl3 has a tetrahedral pyramidal or trigonal pyramidal molecular geometry and ammonia like electron geometry according to the VSEPR theory. It undergoes sp3 hybridisation which results in tetrahedral electron pair geometry and Trigonal pyramidal molecular geometry. Therefore it has 5 electrons in its outermost shell.
The central P atom has one lone pair of electrons and three bond pairs of electrons. Determine the electron geometry eg molecular geometry mg and polarity of PCl3. The central P atom has one lone pair of electrons and three bond pairs of electrons.
Asked Aug 6 2019 in Chemistry by scienceislife. The rate of removal of PCl3 ie. The is mainly due to the disproportionate influence or greater repulsion of the phosphorus lone pair which makes it deviate from the ideal angle of 109 o.
Study PCL3 Molecular Electron Geometry Lewis Structure Bond PCL3 Molecular Electron Geometry Lewis Structure Bond PCl3 Molecular Geometry Shape and Bond Angles YouTube. The process is controlled by the boiling temperature which is determined by the phosphorus content. Why is PCl3 tetrahedral.
Eg tetrahedral mg trigonal pyramidal polar E. Pcl3 Lewis Structure Electron Geometry What is the molecular geometry of PCL3. Need help with chemistry.
The shape of a PCl3 molecule is Trigonal pyramidal. Because the core central atom phosphorus has three P-Cl bonds with the surrounding three chlorine atoms in the bottom of tetrahedral geometry. To attain stability each of the 5 Chlorine atoms will form a bond with Phosphorus.
It undergoes sp3 hybridisation which results in tetrahedral electron pair geometry and Trigonal pyramidal molecular geometry. The production rate is equivalent to the feed rate of phosphorus and chlorine. A 1trigonal planar 2tetrahedral 3trigonal pyramidal b where x represents the outer atoms in each molecule.
PCl3 Phosphorus Trichloride. Why is BCl3 electron deficient. Eg tetrahedral mg bent nonpolar B.
Phosphorus having atomic number 15 has an electron composition of 2 8 5. In PCl3 there are no pi electrons there are no double bonds. The electron geometry for th.
Determine the electron geometry eg molecular geometry mg and polarity of pcl3. The vapors are fractionated as reflux takes place and the PCl3 condenses in air-cooled condensers. As boron has three valence electrons it forms 3 single bonds with chlorine In total the boron atom gives 6 electrons in the outermost.
Why is PCl3 tetrahedral. So PCl3 does not exhibit resonance. Pcl3 structure lewis electron dot bond phosphorus geometry molecular angles hybridization trichloride shape draw atom central many lewis structure molecular electron hybridization bond pcl3 angles geometry shape polarity phosphorus dot angle diagram central atom bf3 polar nonpolar.
Determine the polarity of. Eg linear mg linear polar D. This is due to PCl3 being sp3 hybridized.
This is due to PCl3 being sp3 hybridized. PCl 3 Molecular Geometry And Bond Angles. A quick explanation of the molecular geometry of pcl3 including a description of the pcl3 bond based on vsepr theory valence shell electron pair repulsion theory the electron clouds on atoms and lone pair of electrons polar molecules tutorial.
Read More About Hybridization of Other Chemical Compounds. The shape of a PCl3 molecule is Trigonal pyramidal. Looking at the PCl 3 molecular geometry it is trigonal pyramidal with a bond angle of approx.
Chlorine has 7 electrons in its outermost shell owing to its atomic number 17 and resultant placement 287. Yes BCl3 is an electron-deficient compound. Eg trigonal pyramidal mg trigonal pyramidal nonpolar.

Pcl3 Lewis Structure Hybridization Molecular Geometry And Mo Diagram Techiescientist

Hybridization Of Pcl3 Hybridization Of Phosphorus In Pcl3
What Is The Molecular Shape Of Pcl3 Quora
Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization
Https Www Mctcteach Org Chemistry C1020 C1020 Handouts Molecular 20modeling 20v 8 18 Pdf

What Is The Molecular Geometry Of Pcl3 Study Com

Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization

Pcl3 Molecular Electron Geometry Lewis Structure Bond Angles And Hybridization

Pcl3 Lewis Structure Hybridization Molecular Geometry And Mo Diagram Techiescientist
Your Turn A Central Atom Has Two Lone Pair Of Electrons Around It And Two Single Bonds To Other Atoms What Is The Electron Pair Geometry Around The Central Ppt Download

Pcl3 Molecular Geometry Shape And Bond Angles Youtube

How Can The Molecular Geometry Of Phosphorus Trichloride Be Described Quora

Pcl3 Lewis Structure And Molecular Geometry Youtube

Pcl3 Lewis Structure Hybridization Molecular Geometry And Mo Diagram Techiescientist

How Many Lone Pair Electrons Are There In Pcl3 Quora
What Is The Molecular Shape Of Pcl3 Quora
Your Turn A Central Atom Has Two Lone Pair Of Electrons Around It And Two Single Bonds To Other Atoms What Is The Electron Pair Geometry Around The Central Ppt Download

Hybridization Of Pcl3 Hybridization Of Phosphorus In Pcl3

What Is The Molecular Shape Of Pcl3 Quora