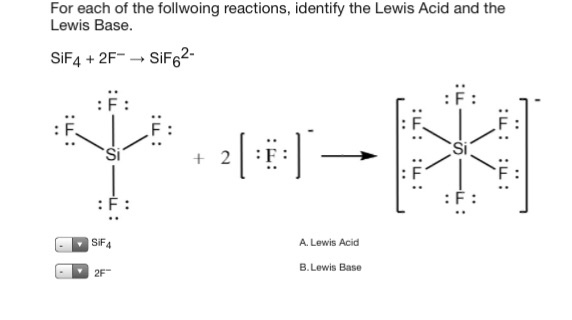
Sif4 Lewis Acid Or Base
Molecules where the central atom can have more than 8 valence shell electrons can be electron acceptors and thus are classified as Lewis acids eg SiBr4 SiF4. Is SiCl4 A Lewis Acid Or Base.

Is Sif4 A Lewis Base Or Lewis Acid
SF4 has a lone pair on the S.

Sif4 lewis acid or base. Is SiF 4 a lewis base or lewis acid. SiF4 2F- SiF62- SF4 F- SF5- Its taking that negative charge electrons from the F-Be careful not to confuses these with the Bronsted-Lowery acids. Hence Br2 is considered to be a soft Lewis acid.
Search More info Main menu. B C2H4 ExplanationIn BF3 and FeCl3 molecules the central atoms have incomplete octet and in SiF4 the central atom has empty d-orbitals. An atom ion or molecule with an incomplete octet of electrons can act as an Lewis acid eg BF3 AlF3.
Now in case of SiF4 molecule 3d- orbitals are empty hence it can accept electron pair from Lewis base eg. Lewis acid can accept a pair of electron from Lewis base. But here according to Low Space Lows base is a species that has ability to do need.
But it has ability to accept the borough of electron in thes vacant orbital. The correct option is. The equilibrium constant for the reaction between a metal ion and ligands.
Hence according to Lewis concept these are Lewis acids. SiF4 can act as a Lewis acid because Si can expand its octet. Lewis acid can accept a pair of electron from Lewis base.
Can OH- function as a Lewis base. Na is not a Lewis acid. F- to give SiF6 2- ion.
Thus 4e3c bonds are sufficiently accessable and stable for silicon in ceSiF4 to act as a Lewis acid. Most compounds which are Lewis acids require an activation step before production of the adduct with Lewis base. Now in case of SiF4 molecule 3d- orbitals are empty hence it can accept electron pair from Lewis base eg.
Silicon is larger than carbon and better able to stabilise 4e3c bonds due to its lower electronegativity. On the other hand such process is not possible for compounds formed by 2nd period elements. Lewis acid is an electron pair acceptor.
Oh- donates a pair of its electrons. Hence they can not act as Lewis Acid e. Let us help you simplify your studying.
Learn this topic by watching Lewis Acid and Base Concept Videos All Chemistry Practice Problems Lewis Acid and Base Practice Problems. Can Oh function as a Lewis base. F- to give.
Cu2 accepts electron pairs in order to make complexes. SiF4 2 F SiF62. Depending on the nature of ceX- the pentacoordinated ceSiF4X- species may either break down under liberation of either ceF- mathrmS_NSi substitution or ceX-.
Learn vocabulary terms and more with flashcards games and other study tools. Hard acids have small acceptor atoms of low polarisability and. Now in case of SiF4 molecule 3d- orbitals are empty hence it can accept electron pair from Lewis base eg.
Elektra and the years of boredom is makes rebound with which and has orbital empty so it dont reach in electing toe Act as a low space. SARL K FOOD COMPANY. Similarly popular cases are the aluminum trihalides that are visible widely as Lewis acids.
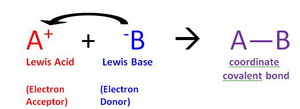
Start studying Lewis Acids and Bases. A Lewis acid is a species that can accept an electron pair whereas a Lewis base has an electron pair available for donation to a Lewis acid. Provide An Example Of A Reaction.
Heres how SiF4 acts like a Lewis acid. Based on the Lewis definition. Ralph Pearson classified all Lewis acids and bases as hard and soft acids and bases.
Is C2H4 a Lewis acid or base. Most compounds which are Lewis acids require an activation step before production of the adduct with Lewis base. Lewis base is an electron pair donor.
In many cases the Lewis acid can bind two Lewis base a popular example being the formation of the hexafluorosilicate. Is Na a Lewis acid or base. F- to give SiF6 2- ion.
Complex ions are examples of Lewis acid-base adducts and comprise central metal atoms or ions acting as Lewis acids bonded to molecules or ions called ligands that act as Lewis bases. Br2 and Br are soft Lewis acids and Br- has properties in between soft bases and hard bases. Soft acids have large acceptor atoms of low positive charge high polarisability and low electronegativity.

Chemistry 73 Which Of The Following Fluoro Compounds Is Most Likely To Behave As A Lewis Base 1 Cf4 2 Sif4 3 Bf3 4 Pf 74 Which Of The Following Pairs Of Ions Is Isoelectronic Ctural
Solved The Uorides Bf3 Aif3 Sif4 And Pf5 Are Lewis Acids The All Form Very Stable Uoroanions When Treated With Lithium Uoride In Contrast The Course Hero

Ch 4 Acids And Bases 1 Bronstedlowry Definitions

Solution Which Of The Following Is A Lew Chemistry
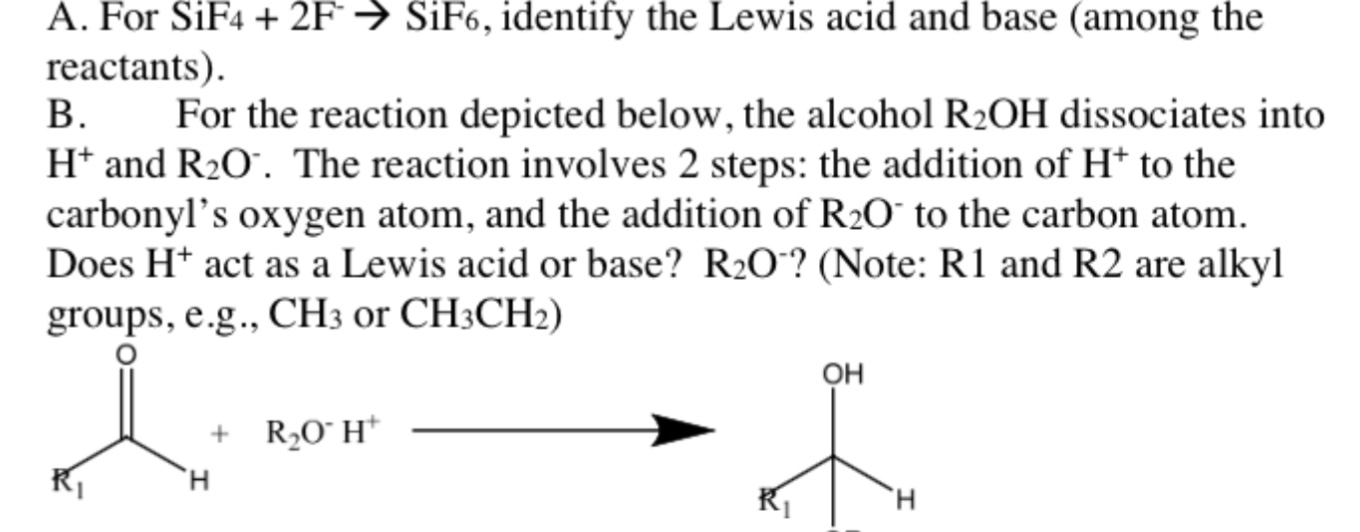
A For Sif4 2f Sif6 Identify The Lewis Acid And Chegg Com

Which Are Of The Following Are Lewis Acids Cheek Chegg Com

Is Sif4 A Lewis Base Or Lewis Acid

Which Of The Following Is Not A Lewis Acid A Sif4 B C2h4 C Bf3 D F
Chem Molecular Shape Molecular Geometry Scientific Tutor
What Is The Lewis Structure Of Sif4 And How Does It Compare To That Of Nitrogen Quora

What Is The Lewis Structure Of Sif4 And How Does It Compare To That Of Nitrogen Quora
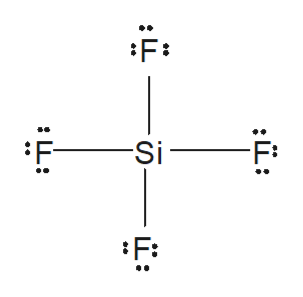
Answered From The Lewis Diagram Of Sif4 Bartleby
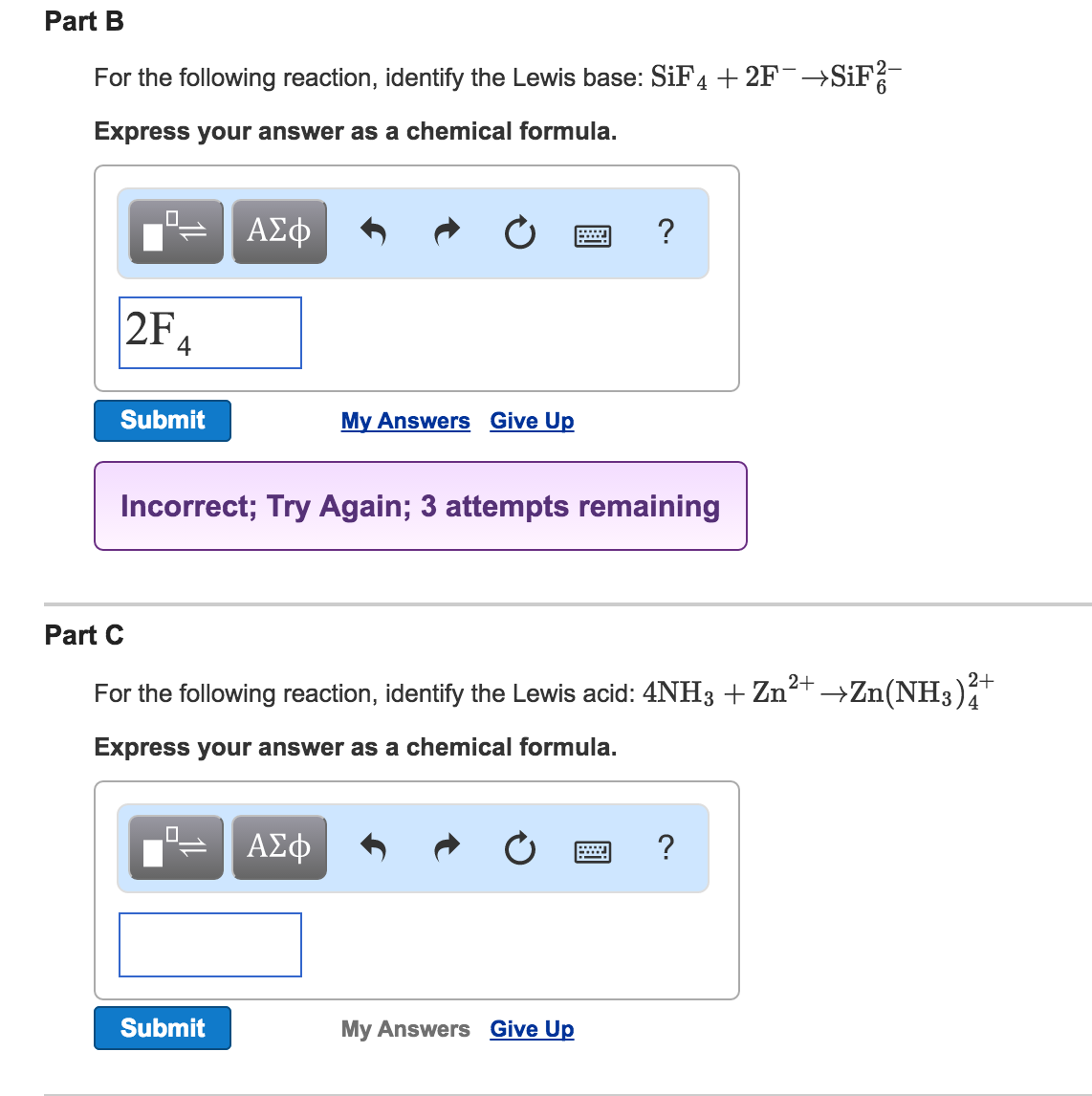
Part B For The Following Reaction Identify The Lewis Chegg Com
For Each Of The Following Reactions Identify The Chegg Com
Why Does Sif4 Act As A Lewis Acid Example

Lewis Acid And Base Concept For Each Of The Following Chegg Com