Sulfur Tetrachloride Lewis Structure
Sulfur tetrachloride is an inorganic compound with chemical formula SCl 4. S does not follow the octet rule.

H2s Lewis Structure Dihydrogen Sulfide In 2021 Lewis Molecules Chemical Formula
These are arranged in a trigonal bipyramidal shape with 102 F-S-F bond angles between the equatorial fluorine atoms and 173 between the axial fluorine atoms.

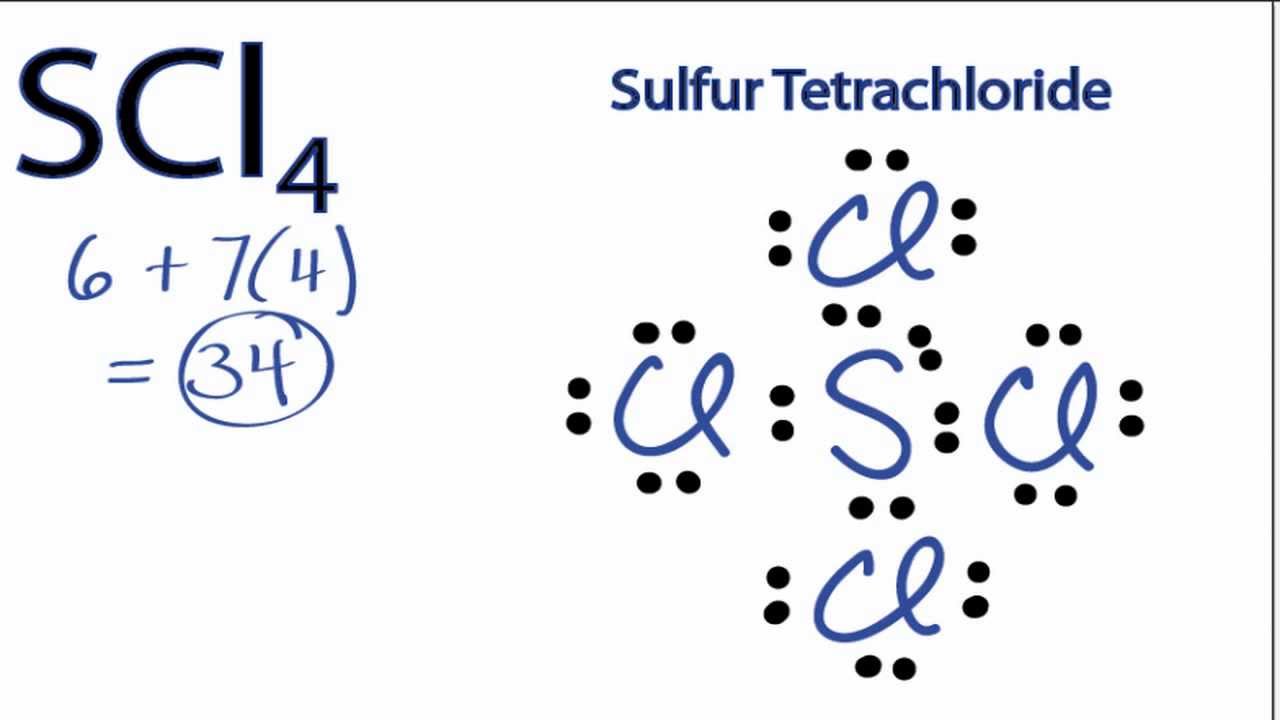
Sulfur tetrachloride lewis structure. There are four Chlorines. It is obtained by treating sulfur dichloride with chlorine at 193 K. The total number of electrons in the Lewis structure Sulfur Tetrachloride SCl 4 must equal the total number of valence electrons.
It has only been obtained as an unstable pale yellow solid. 70 More Lewis Dot Structures. It is a see-saw shape with S at the center.
Sulfur tetrachlorideSCl4 has the composition of one sulfur and four chlorine atoms. Put the least electronegative atom in the center. One of the three equatorial positions is occupied by a nonbonding lone pair of electrons.
NOCl CF 2 Cl 2 HCN. Sulfur is the least electronegative well put that at the center and then well put the Chlorines around the outside. Sulfur having valence electrons in the 3rd energy level will also have access to the 3d sublevel thus allowing for more than 8 electrons.
Then well go around the outer atoms. Geometry electron sulfur tetrachloride hybridization scl pair lewis scl4 structure draw lone hybridisation bonds. A step-by-step explanation of how to draw the SF4 Lewis Dot Structure Sulfur tetrafluorideFor the SF4 structure use the periodic table to find the total n.
H 2 S NCl 3 OH -. Drawing and predicting the SCl4 Lewis Structure is very easy by following the given method. SF 4 is dsp 3 hybridized and contains 1 lone pair and 4 bonding pairs of valence electrons around the sulfur.
Answer to Sulfur tetrachloride SCl_4 Lewis Structure. Of sulfurs total of six valence electrons two form a lone pair. This is the SCl4 Lewis structure.
Chlorine has 7 but there are four Chlorines. This is the SCl4 Lewis structure. XeO 2 F 2.
The structure of SF 4 can therefore be anticipated using the principles of VSEPR theory. Sulfur in SF 4 is in the formal 4 oxidation state. Shape vsepr using sicl4 molecular structure theory molecule predict following ph3 h2s chemical bonding discuss bond tetrahedral pairs four ii.
Steps for Writing Lewis Structures. SULFUR TETRAFLUORIDE is a highly toxic and corrosive gas. It will hold more than 8 electrons.
This is the SCl4 Lewis structure. So 6 plus 28 is 34 total valence electrons. In the Lewis structure for SCl4 we well need to put 10 valence electrons on the central Sulfur atom.
The corresponding SF 4 is a stable useful reagent. Drawing the Lewis Structure for SCl. View Live Sulfur tetrafluoride has 5 regions of electron density around the central sulfur atom 4 bonds and one lone pair.
What is the molecular geometry of sulfur tetrachloride. Chlorine has 7 but there are four Chlorines. Put two electrons between atoms to form a chemical bond.
You might want to put a double bond on the Chlorine Cl atom but Cl doest normally form double bonds due to its high electronegativity. Write the name and formulas for the following compounds covalent compounds and lewis structure practice write the formulas for the following covalent compounds. There are four Chlorines.
Hydrogen peroxide H_2O_2 Lewis. H always goes outside. 10 12 and 32.
Sulfur is the least electronegative well put that at the center and then well put the Chlorines around the outside. Shape sicl4 molecular sf4 brf3 ph3 structure bonding dear chemical molecule tetrahedral chemistry sicl student. Sicl4 Lewis Structure Vsepr The Shapes Of Molecules Phosphorus trichloride Wikipedia ZinnIV chlorid Wikipedia SiF4 Lewis Structure How to Draw the Dot Structure for.
Sulfur has 6 valence electrons. Find the total valence electrons for the molecule. Sulfur has 6 valence electrons.
4 Sulfur is in Period Four on the periodic table and can hold more than eight valence electrons. On contact with water steam or mineral acids it decomposes and produces toxic and highly irritating fumes. Here in this post we described step by step to construct Lewis structure of SCl4.
So 6 plus 28 is 34 total valence electrons. Scl4 lewis structure sulfur tetrachloride draw. When heated to decomposition it emits very toxic fluoride and sulfur oxides fumes Lewis 3rd ed 1993 p.
Atom Valence S 6 e Cl 7 e Cl 7 e Cl 7 e Cl 7 e Total 34 e. Geometry molecular sicl4. The Lewis structure for SCl4 has 34 valence electrons available to work with.

Lewis Dot Structure Easy Hard Science
Lewis Structure Of Sf4 And Hybridization Of Sf4 Sulfur Tetrafluoride Lewis Structures

Lewis Dot Structure Easy Hard Science

Sf4 Lewis Structure How To Draw The Lewis Structure For Sf4 Youtube

So2 Lewis Structure Chemistry Worksheets Electron Configuration Chemistry Notes

Lewis Dot Structure Easy Hard Science

Xef4 Lewis Structure How To Draw The Lewis Structure For Xef4 In 2021 Lewis Octet Rule Noble Gas

Scl4 Lewis Structure How To Draw The Lewis Structure For Sulfur Tetrachloride Youtube

Sef4 Lewis Structure How To Draw The Lewis Structure For Sef4 Youtube

Lewis Dot Structure Easy Hard Science

Sf4 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

Ch2o Lewis Structure Methanal Or Formaldehyde In 2021 Methanal Molecules Lewis

Lewis Structure For Ch4 Practices Worksheets Graphing Linear Equations Chemistry Worksheets
Sf4 Molecular Geometry Lewis Structure Bond Angles And Polarity

Lewis Dot Structure Easy Hard Science

Lewis Dot Structure Easy Hard Science

Ccl4 Lewis Structure Carbon Tetachloride In 2021 Carbon Molecule Molecules Lewis

Is Bf3 Polar Or Non Polar Boron Trifluoride In 2021 Boron Atom Molecules Chemical Formula
Scl4 Lewis Structure How To Draw The Lewis Structure For Sulfur Tetrachloride Youtube