What Is The Correct Lewis Structure For Co2 Quizlet
Determine the number of electrons that the four elements below require to obtain an octet of electrons. 3 4 2 2 c.

Pf3 Lewis Structure How To Draw The Lewis Structure For Pf3 Youtube
Which one of the following substances should exhibit hydrogen bonding in the liquid state.

What is the correct lewis structure for co2 quizlet. Covalent bonds usually involve two nonmetals and ionic bonds usually involve a metal and a nonmetal. What is the correct Lewis structure for CO2. What is the correct Lewis structure for N2.
Nitrogen oxygen carbon fluorine a. We review their content and use your feedback to keep the quality high. 1 3 2 4 b.
Chemistry Common Ions. 10 14 10 c. In order to complete the octets for all of the atoms in the structure.
What Do You Know About Lewis Structures. C O. 10 14 14 b.
There are three. Correct BCl3 Br2 H2 CO2. Which statement correctly describes the structure of the whole.
Arrange the following substances in order of increasing boiling point. It has six electrons in valence shell. Lets draw the structure.
D N N. What is the correct Lewis structure for CO2 quizlet. 5 6 4 7.
Why does the correct Lewis structure CO2 involve a double bond between each of the oxygen atoms and carbon atoms. More Lewis Structure Quizzes. But we have two of them.
4 3 5 2 e. Lewis Structures 20 30 21 40 Linear. E none of the above.
What is the Lewis structure for SF 2. Total valence electrons given by carbon atom 4. To complete the octet for carbon.
Which of the elements you tested doesnt have a characteristic metallic appearance. Learn vocabulary terms and more with flashcards games and other study tools. The Lewis model predicts that the formula for a compound between fluorine and calcium is.
The Lewis structure for CO2 has a total of 16 valence electrons. What Do You Know About Lewis Structures. Which is the correct molecular structure for CO2.
Who are the experts. Chem30A Chapter 5 Quiz. Tetrahedral 20 Linear 30 Trigonal Planar.
So well put. Whether you call them Lewis structures electron dot structures Lewis electron dot structures Lewis dot structures Lewis. Choose from 500 different sets of lewis structures flashcards on Quizlet.
Which of the following molecular compounds would have a Lewis structure that contains 10 electron dots. And then Oxygen is in group 6 or 16. Total number of electrons of the valance shells of CO 3 2-Carbon is located at group 4 in the periodic table.
The initial VSEPR shape for the CO2 molecule is Tetrahedral. He N2 CH3Cl. CH3OH He CH3Cl and N2 CH3OH He CH3Cl N2 He N2 CH3OH CH3Cl N2 He CH3OH CH3Cl He N2 CH3Cl CH3OH CH3Cl He N2 CH3OH.
16 14 10 e. So carbon has four electrons in its valence shellOxygen is located at 6 th group. The Lewis structure for carbon monoxide is.
Write the correct Lewis dot structure for O2. Drawing correct lewis structure is important to draw resonance structures of CO 3 2-correctly. 14 26 14 d.
On the periodic table Carbon is in group 4 or 14 sometimes. Were going to do the Lewis structure for CO2 Carbon dioxide. Furthermore What is the correct Lewis structure for CO2 quizlet 19 When calculating the number of electrons for the Lewis structure of a polyatomic ion subtract one electron for each negative charge.
What does the Lewis structure for Carbo What is the electron-dot diagram for Br What is the correct. If we talk about ions it simply means that an atom has gained of lost electrons by transfer from or to other atoms during the formation of an ionic bond. That means its going to go at the center.
See the answer See the answer See the answer done loading. This problem has been solved. A CaF B Ca2F C CaF3 D CaF3 E None of the above.
Show transcribed image text Expert Answer. Log in Sign up. Carbon is the least electronegative.
20 The correct Lewis structure for CO2. Experts are tested by Chegg as specialists in their subject area. C D N N.
20 The correct Lewis structure for CO2 shows that the molecule contains two double bonds. None of the above. Rate Of Reaction.
Start studying intro to chemistry chap 10. Which of the following statements contrasting covalent bonds and ionic bonds is correct. 19 When calculating the number of electrons for the Lewis structure of a polyatomic ion subtract one electron for each negative charge.
So lets multiply that together there. 3 2 4 1 d. Carbon C is the least electronegative atom in the CO2 Lewis structure and therefore should be placed at the center of the structure.
So we have 12 plus 4 16 total valence electrons. PH3 H2 H2S CH4 NH3. A 4 lone pairs and 1 bonding pair B 4.
22 26 14. A N - N B -.

Lungs Flashcards Quizlet Human Anatomy And Physiology Human Body Anatomy Anatomy And Physiology

Chapter 8 Section 3 Study Guide Flashcards Quizlet

Exam 3 Homework Set Week 11 Flashcards Quizlet

Co2 Lewis Structure Easy Hard Science

The Bronchial Tree Note How Each Main Bronchus Enters A Lung And Then Branches Into Smaller And Sma Biology Forums Gallery Legkie

Close Up Of Tracheal Cilia Human Respiratory And Excretory Systems Flashcards Quizlet Respiratory System Respiratory Excretory System

Lewis Dot Structure Practice Flashcards Quizlet

Ted Talk On The Chemistry Of Baking Baking Science Food Science Chemistry
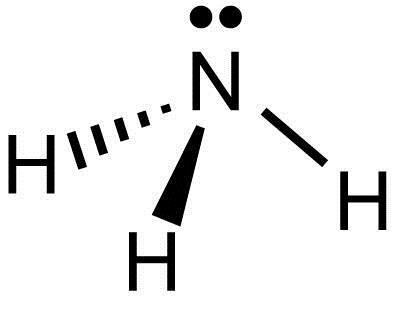
What Is The Lewis Structure Of Nh3 Socratic

C3h4 Lewis Structure How To Draw The Lewis Structure For Ch3cch Youtube

Chemistry 114 Chapter 10 Flashcards Quizlet
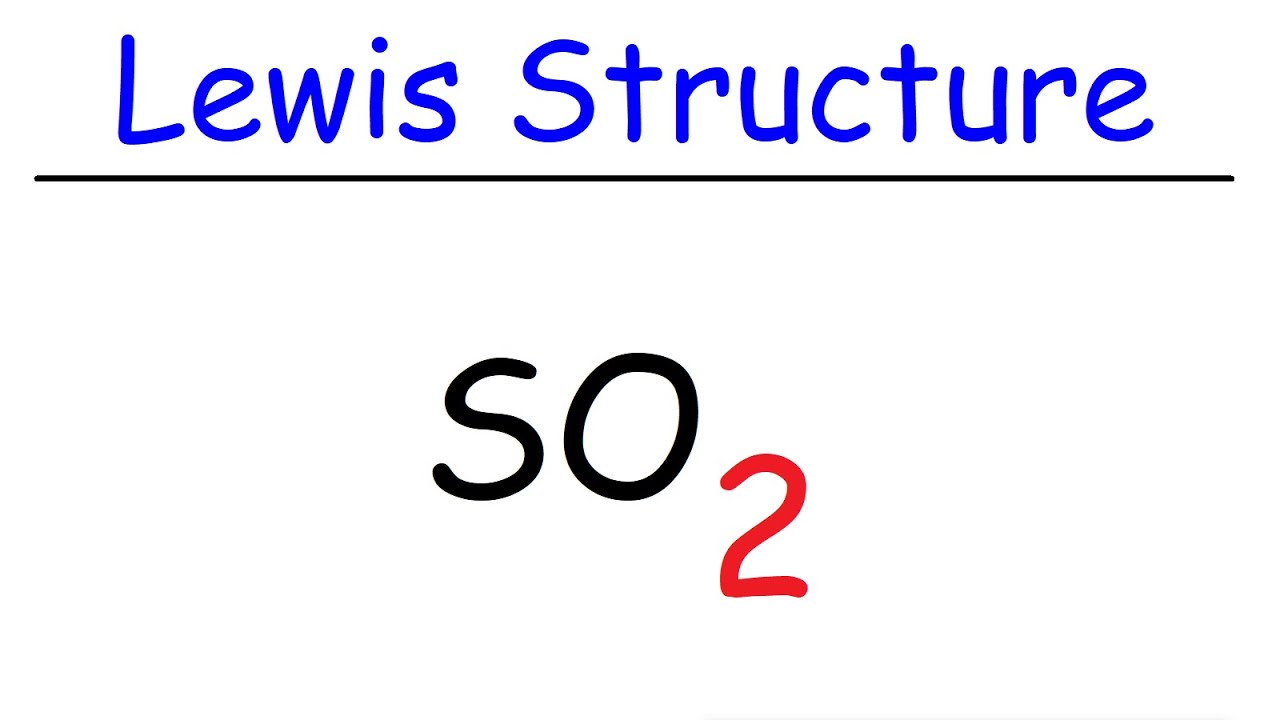
So2 Lewis Structure Sulfur Dioxide Youtube
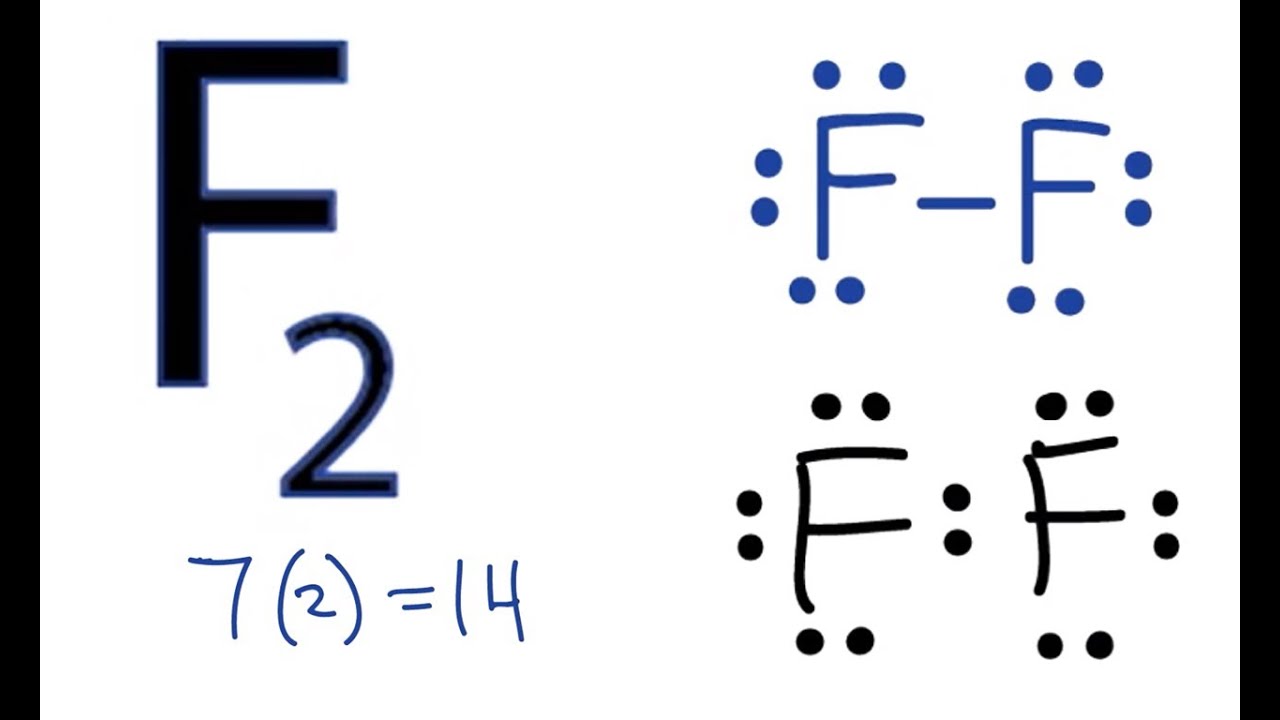
F2 Lewis Structure How To Draw The Lewis Dot Structure For F2 Youtube

Co2 Lewis Structure Easy Hard Science

Co2 Lewis Structure Carbon Dioxide In 2021 Carbon Dioxide Lewis Molecules

Unit 4 Review Objectives 11 19 Flashcards Quizlet

Chemistry 2 3 Lewis Structures Flashcards Quizlet

