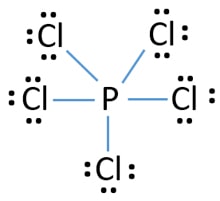
Why Pcl5 Is Lewis Acid
It turns out that phosphorus very readily accepts electrons from other molecules which is why it generally behaves as a Lewis acid. Why is PCl5 a Lewis acid.

Is Pcl5 Polar Or Nonpolar Techiescientist
Our website has a team of professional writers who can help you write any of your homework.
Why pcl5 is lewis acid. What you can read next. The phosphorus in PCl 5 readily accepts electrons from other molecules. For PCl_5 to be a Lewis acid it would have to react in such a way that it accepted a lone pair from some other atom or molecule from a Lewis base.
For PCl_5 to be a Lewis acid it would have to react in such a way that it accepted a lone pair from some other atom or molecule from a Lewis base. Environmental Scan Paper. Therefore it is considered as a Lewis acid.
The phosphorus in PCl 5 readily accepts electrons from other molecules. For PCl_5 to be a Lewis acid it would have to react in such a way that it accepted a lone pair from some other atom or molecule from a Lewis base. According to the Lewis definition of a compound isnt acidic or basic until it does something.
According to the Lewis definition of a compound isnt acidic or basic until it does something. It turns out that phosphorus very readily accepts electrons from other molecules which is why it generally behaves as a Lewis acid. If you have searched a question and bumped into.
According to the Lewis concept acid is the substance that accepts a lone pair of electrons since it has empty orbitals in the valence shell. In other words you cant just say that X compound is a Lewis acid unless youve seen it act as an acid in some chemical reaction in which case youd say that X compound is a Lewis acid. Type of paper.
We are a professional custom writing website. It turns out that phosphorus very readily accepts electrons from other molecules which is why it generally behaves as a Lewis acid. Who We Are.
For PCl_5 to be a Lewis acid it would have to react in such a way that it accepted a lone pair from some other atom or molecule from a Lewis base. For PCl_5 to be a Lewis acid it would have to react in such a way that it accepted a lone pair from some other atom or molecule from a Lewis base. Place an Order Now.
In other words you cant just say that X compound is a Lewis acid unless youve seen it act as an acid in some chemical reaction in which case youd say that X compound is a Lewis acid in this particular reaction. For PCl_5 to be a Lewis acid it would have to react in such a way that it accepted a lone pair from some other atom or molecule from a Lewis base. Click to share on Twitter Opens in new window Click to share on Facebook Opens in new window.
According to the Lewis concept acid is the substance that accepts a lone pair of electrons since it has empty orbitals in the valence shell. It turns out that phosphorus very readily accepts electrons from other molecules which is why it generally behaves as a Lewis acid. With our discounted prices and over.
In other words you cant just say that X compound is a Lewis acid unless youve seen it act as an acid in some chemical reaction in which case youd say that X compound is a Lewis acid. It turns out that phosphorus very readily accepts electrons from other molecules which is why it generally behaves as a Lewis acid. Pages 550 words Approximate.
Our hand chosen and dedicated team of essay experts are here just for you. Continue to order Get a quote. Calculate your paper price.
According to the Lewis definition of a compound isnt acidic or basic until it does something. Why are flowing plants considered by many botanists to be the. Why is PCl5 Lewis acid.
For PCl_5 to be a Lewis acid it would have to react in such a way that it accepted a lone pair from some other atom or molecule from a Lewis base. It turns out that phosphorus very readily accepts electrons from other molecules which is why it generally behaves as a Lewis acid. For PCl_5 to be a Lewis acid it would have to react in such a way that it accepted a lone pair from some other atom or molecule from a Lewis base.
It turns out that phosphorus very readily accepts electrons from other molecules which is why it generally behaves as a Lewis acid. They will write. It turns out that phosphorus very readily accepts electrons from other molecules which is why it generally behaves as a Lewis acid.

Phosphorus Pentachloride Wikiwand

Are Silicon Tetrachloride And Phosphorus Pentachloride Lewis Acid Or Lewis Base Quora

Is Pcl5 An Acid Or A Base Quora
Is Pcl5 Polar Or Nonpolar Phosphorous Pentachloride Youtube
What Is The Structure Of Pcl5 Quora
Draw The Lewis Structure Of Pcl5 And Sf6 Sarthaks Econnect Largest Online Education Community

Phosphorus Pentachloride Wikiwand
Are Silicon Tetrachloride And Phosphorus Pentachloride Lewis Acid Or Lewis Base Quora
What Is The Lewis Structure Of Pcl5 Quora
Explain The Structure Of Pcl3 And Pcl5 Chemistry Topperlearning Com C9yvmoxx

Pcl5 Lewis Structure Molecular Geometry Hybridization And Mo Diagram Techiescientist

Reaction Of Alcohols With Pcl5 And Pcl3 Chemistry Stack Exchange
The Chemical Thesaurus Reaction Chemistry Database

How To Draw The Lewis Structure Of Pcl5 Phosphorus Pentachloride Youtube