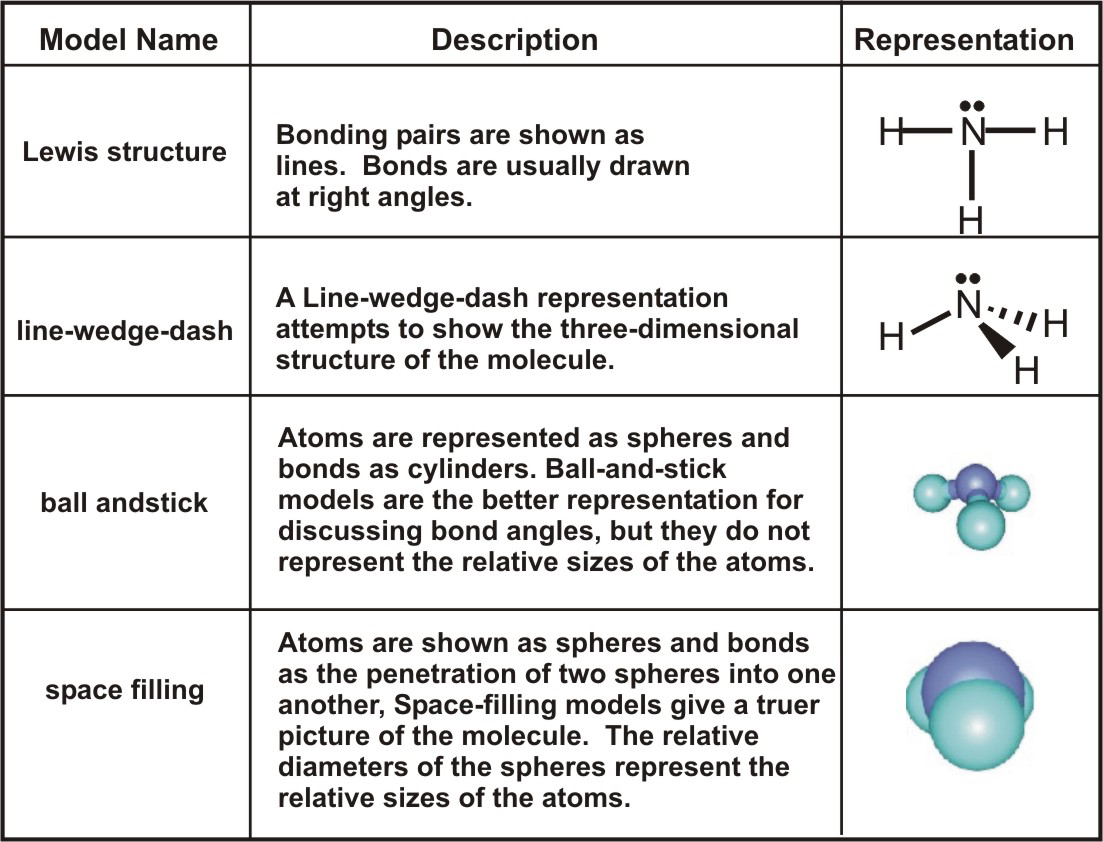
How To Determine Bond Angle In A Molecule
Name the electron-group geometry. Bond angle and bond length are the two important parameters which determine the shape and size of a molecule.
Chem Bond Angle Scientific Tutor
Then the angle between the bonds of any 2 of the non central atoms with the central atom is the bond angle.
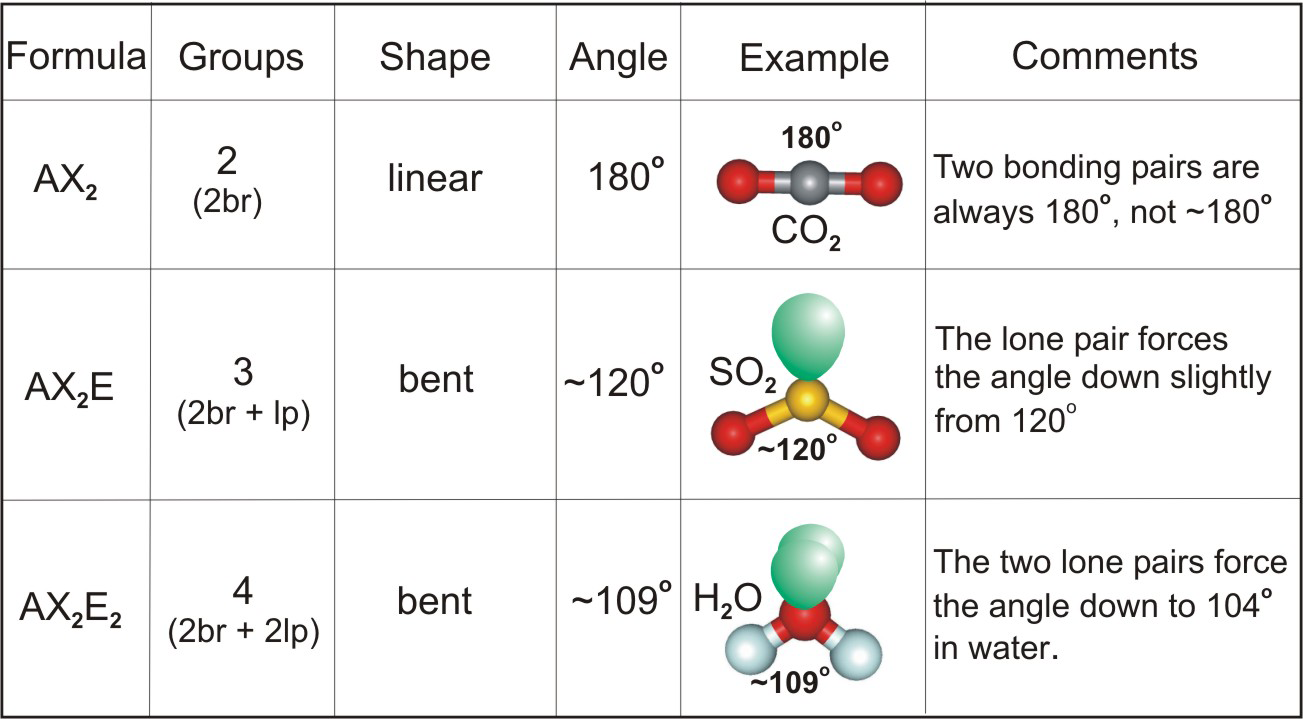
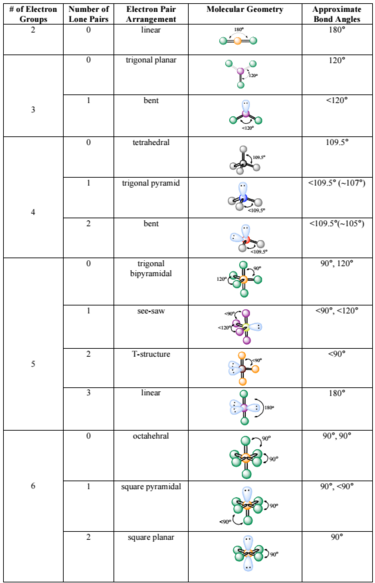
How to determine bond angle in a molecule. They always range from 100- 180 degrees. Distance x2-x12 y2-y12 z2-z12. Count the number of electron groups and identify them as bond pairs of electron groups or lone pairs of electrons.
How do I determine the bond angle in a molecule. For many complex eg. So if there is a molecule ABC in which both B and C are bonded attached to A then the angle between the lines AB and AC is the bond angle.
Bioorganic molecules this fails but there are two afaik last hopes to help solveprove structures if they dont crystallise and the usual NMR. Draw the Lewis Structure. The coordinates can be seen.
The most common method of measuring bond lengths in solids is by analysis of the diffraction or scattering of X-rays when they pass through the regularly-spaced atoms in the crystal. As shown in the diagram the molecule can be inscribed in a cube with the tetravalent atom eg. Click on Set Bond Angle and enter a value in degrees for the bond angle.
The angle between the two bonds is known as the bond angle of that molecule. The formula for a distance between two points in three dimensional space is. This short chemistry video shows you how to determine the bond angle in the different shapes of moleculesPart 1- Determining Shapes of MoleculesPart 2- Dete.
Click on Editon the menu bar. Use the steric number and VSEPR theory to determine the electron domain geometry of the molecule. Assume that you must determine the bond angles in BF3.
For gaseous molecules neutron- or electron-diffraction can also be used. In a molecule of H 2 O there are 2 lone pairs. Assume that you must determine the bond angles in BF 3 B is less electronegative than F so B becomes the central atom If we have three F atoms that means that we are going to use all three electrons from the B.
Steps Used to Find the Shape of the Molecule. Tetrahedral bond angle The bond angle for a symmetric tetrahedral molecule such as CH4 may be calculated using the dot product of two vectors. The bond angle is.
Bond length is the distance between the nuclei of two bonded atoms whereas bond angle is the angle formed betweeen two adjacent atoms in a molecule. 1 Get the group number of the primary element 2 If its a negative charge add that to the group number from 1 or subtract the positive charge 3 Add the bonded pairs 4 Divide by 2 to get the bonded pairs. Write the Lewis dot structure for the molecule.
People have had different questions about proving the distance between the points on the methane molecule. The same case holds true if there are more than two noon central atoms. The bond angle may get deflected due to presence of the non-bonding pairs of electrons.
Click on the three atoms which define the bond angle the three atoms and the bonds between them will become highlighted. To get the VSEPR. Remember electron groups include.
We know this because there are 2 O-H bonds or 2 hydrogen atoms bonded to the oxygen atom. Use the steric number and VSEPR theory to determine the electron domain geometry of the molecule. Write the Lewis dot structure for the molecule.
For the top two points this would equal 2--22 1-12 0-0. In a molecule of H 2 O there are 2 bonding pairs. The most obvious method is single crystal xray crystallography of course and for simple molecules IR and microwave spectroscopy in the gas phase can be used.
When finding the bond angles in molecules we consider the lone pairs AND the bonding pairs. Use the VSEPR shape to determine the angles between the electron domains. Assume that you must determine the bond angles in BF3.

6 3 Molecular Shape Introductory Chemistry
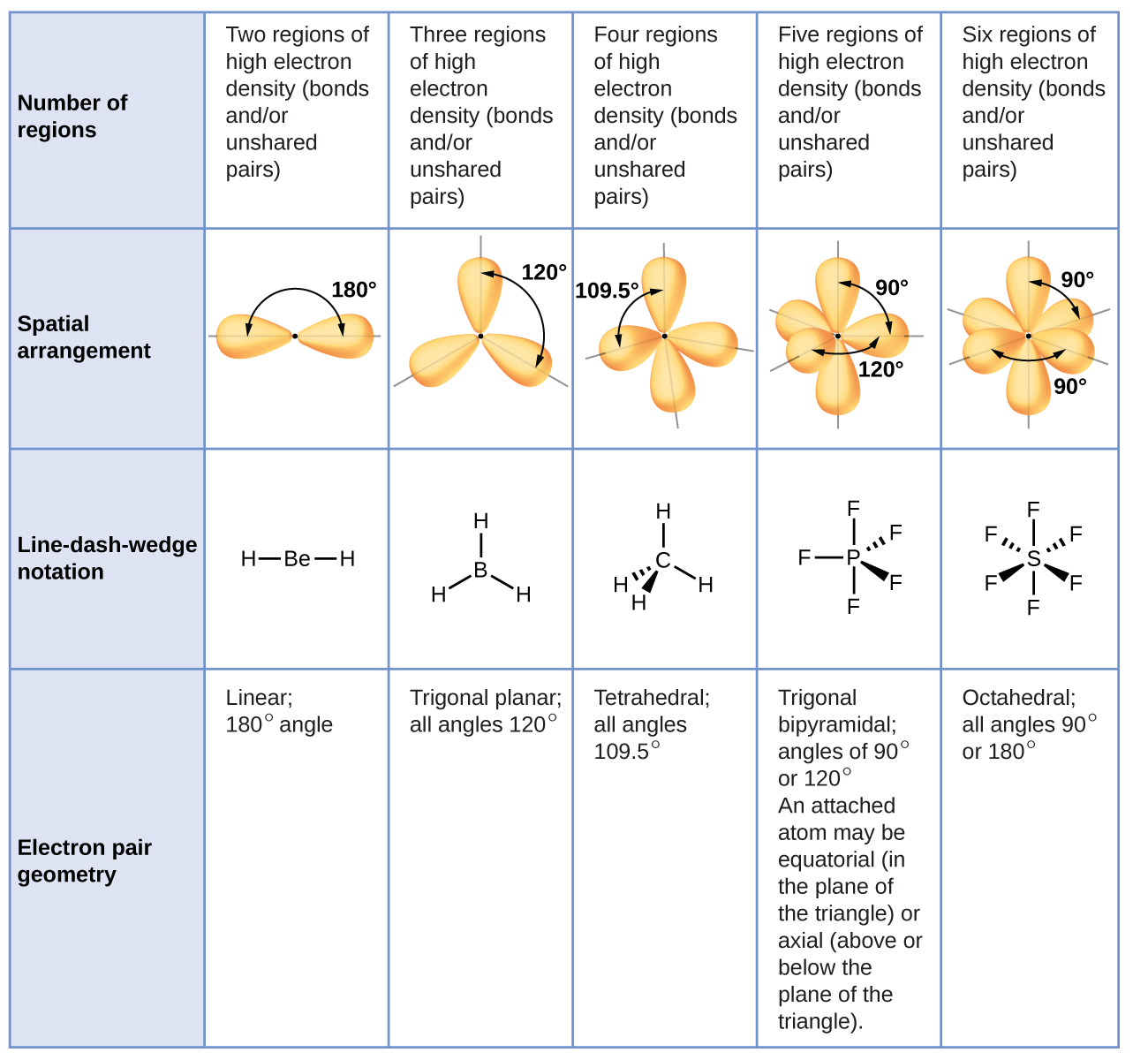
5 2 Molecular Shape Chemistry Libretexts

4 2 7 Predict The Shape And Bond Angles For Species Using Vsepr Theory Youtube
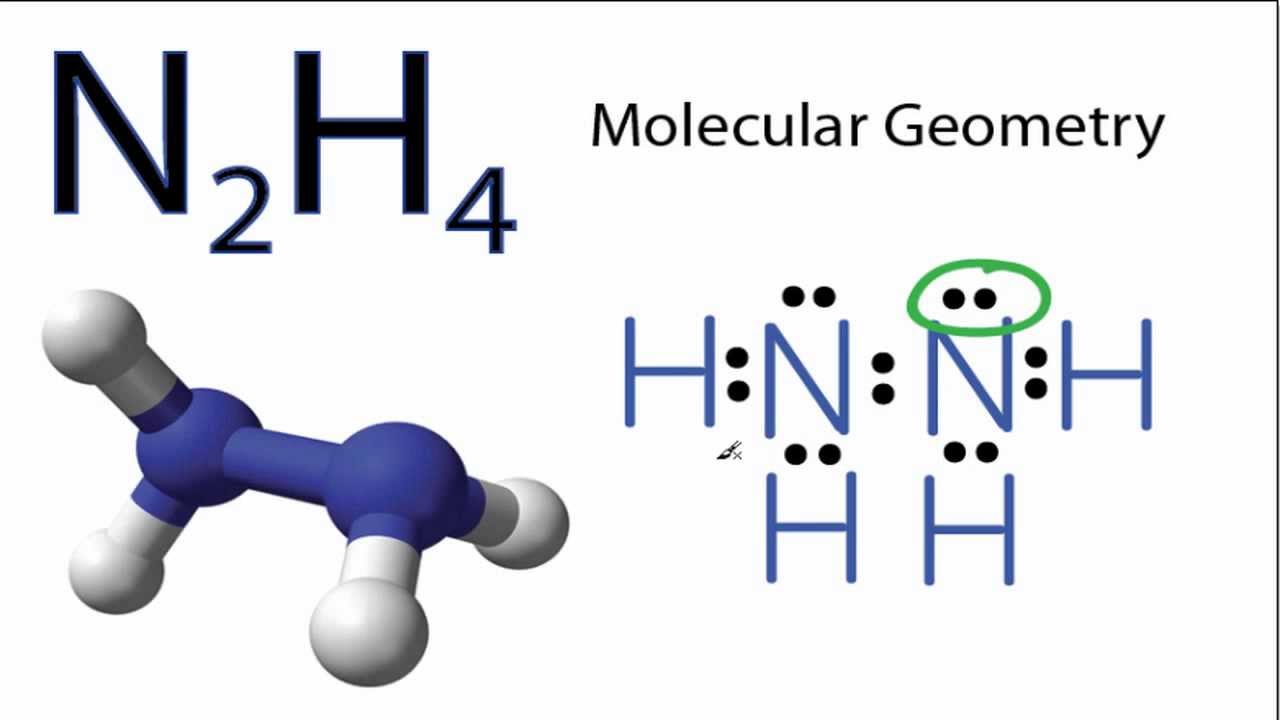
N2h4 Molecular Geometry And Bond Angles Actual Bond Angle Is Less Than 109 5 Degrees Youtube

How To Predict Bond Angles Quora
Chem Bond Angle Scientific Tutor
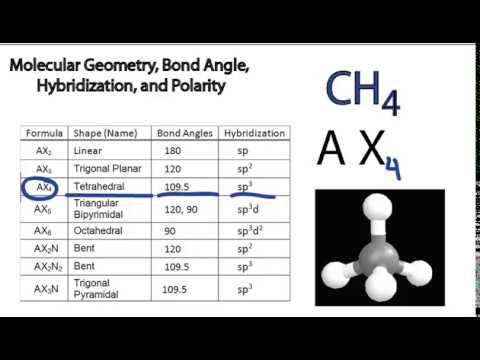
Molecular Geometry Bond Angle Hybridization And Polarity Examples Youtube

What Determines Molecular Shape Bond Angles Angle Formed Between Two Adjacent Bonds On The Same Atom E G Ccl 4 Chapter 9 Molecular Geometry And Bonding Ppt Download

What Determines Molecular Shape Bond Angles Angle Formed Between Two Adjacent Bonds On The Same Atom E G Ccl 4 Chapter 9 Molecular Geometry And Bonding Ppt Download

What Is The Bond Angle Of Of2 Study Com

Molecular Geometry Boundless Chemistry

Predicting Bond Angles Youtube

Bond Angles Tricks To Compare Bond Angle For Different Molecules Jee Iit Neet Csir Net Youtube
Predicting Molecular Shapes Vsepr
How Do We Determine The Bond Angles For Molecular Geometries That Have Lone Pairs Around Them E G Bent Trigonal Pyramidal Seesaw Etc Is It True That These Angles Can Only Be Found